Bordetella bronchiseptica exploits the complex life cycle of Dictyostelium discoideum as an amplifying transmission vector
- PMID: 28403138
- PMCID: PMC5389573
- DOI: 10.1371/journal.pbio.2000420
Bordetella bronchiseptica exploits the complex life cycle of Dictyostelium discoideum as an amplifying transmission vector
Abstract
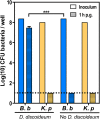
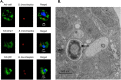
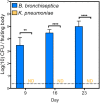
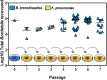
Multiple lines of evidence suggest that Bordetella species have a significant life stage outside of the mammalian respiratory tract that has yet to be defined. The Bordetella virulence gene (BvgAS) two-component system, a paradigm for a global virulence regulon, controls the expression of many "virulence factors" expressed in the Bvg positive (Bvg+) phase that are necessary for successful respiratory tract infection. A similarly large set of highly conserved genes are expressed under Bvg negative (Bvg-) phase growth conditions; however, these appear to be primarily expressed outside of the host and are thus hypothesized to be important in an undefined extrahost reservoir. Here, we show that Bvg- phase genes are involved in the ability of Bordetella bronchiseptica to grow and disseminate via the complex life cycle of the amoeba Dictyostelium discoideum. Unlike bacteria that serve as an amoeba food source, B. bronchiseptica evades amoeba predation, survives within the amoeba for extended periods of time, incorporates itself into the amoeba sori, and disseminates along with the amoeba. Remarkably, B. bronchiseptica continues to be transferred with the amoeba for months, through multiple life cycles of amoebae grown on the lawns of other bacteria, thus demonstrating a stable relationship that allows B. bronchiseptica to expand and disperse geographically via the D. discoideum life cycle. Furthermore, B. bronchiseptica within the sori can efficiently infect mice, indicating that amoebae may represent an environmental vector within which pathogenic bordetellae expand and disseminate to encounter new mammalian hosts. These data identify amoebae as potential environmental reservoirs as well as amplifying and disseminating vectors for B. bronchiseptica and reveal an important role for the Bvg- phase in these interactions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Diavatopoulos DA, Cummings CA, Schouls LM, Brinig MM, Relman DA, Mooi FR. Bordetella pertussis, the causative agent of whooping cough, evolved from a distinct, human-associated lineage of B. bronchiseptica. PLoS Pathog. 2005;1: e45 doi: 10.1371/journal.ppat.0010045 - DOI - PMC - PubMed
-
- Dworkin MS, Sullivan PS, Buskin SE, Harrington RD, Olliffe J, MacArthur RD, et al. Bordetella bronchiseptica infection in human immunodeficiency virus-infected patients. Clin Infect Dis Off Publ Infect Dis Soc Am. 1999;28: 1095–1099. - PubMed
-
- Bendor L, Weyrich LS, Linz B, Rolin OY, Taylor DL, Goodfield LL, et al. Type Six Secretion System of Bordetella bronchiseptica and Adaptive Immune Components Limit Intracellular Survival During Infection. PLoS ONE. 2015;10: e0140743 doi: 10.1371/journal.pone.0140743 - DOI - PMC - PubMed
-
- Cummings CA, Bootsma HJ, Relman DA, Miller JF. Species- and strain-specific control of a complex, flexible regulon by Bordetella BvgAS. J Bacteriol. 2006;188: 1775–1785. doi: 10.1128/JB.188.5.1775-1785.2006 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

