Direct fluorescence detection of VirE2 secretion by Agrobacterium tumefaciens
- PMID: 28403156
- PMCID: PMC5389803
- DOI: 10.1371/journal.pone.0175273
Direct fluorescence detection of VirE2 secretion by Agrobacterium tumefaciens
Abstract
VirE2 is a ssDNA binding protein essential for virulence in Agrobacterium tumefaciens. A tetracysteine mutant (VirE2-TC) was prepared for in vitro and in vivo fluorescence imaging based on the ReAsH reagent. VirE2-TC was found to be biochemically active as it binds both ssDNA and the acidic secretion chaperone VirE1. It was also biologically functional in complementing virE2 null strains transforming Arabidopsis thaliana roots and Nicotiana tabacum leaves. In vitro experiments demonstrated a two-color fluorescent complex using VirE2-TC/ReAsH and Alexa Fluor 488 labeled ssDNA. In vivo, fluorescent VirE2-TC/ReAsH was detected in bacteria and in plant cells at time frames relevant to transformation.
Conflict of interest statement
Figures

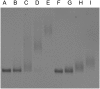





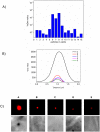
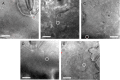
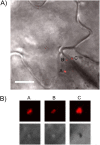
References
-
- Chilton MD, Drummond MH, Merio DJ, Sciaky D, Montoya AL, Gordon MP, et al. Stable incorporation of plasmid DNA into higher plant cells: the molecular basis of crown gall tumorigenesis. Cell. 1977;11: 263–271. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

