Bacterial biofilm under flow: First a physical struggle to stay, then a matter of breathing
- PMID: 28403171
- PMCID: PMC5389662
- DOI: 10.1371/journal.pone.0175197
Bacterial biofilm under flow: First a physical struggle to stay, then a matter of breathing
Abstract
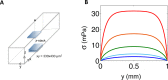
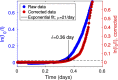
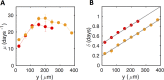
Bacterial communities attached to surfaces under fluid flow represent a widespread lifestyle of the microbial world. Through shear stress generation and molecular transport regulation, hydrodynamics conveys effects that are very different by nature but strongly coupled. To decipher the influence of these levers on bacterial biofilms immersed in moving fluids, we quantitatively and simultaneously investigated physicochemical and biological properties of the biofilm. We designed a millifluidic setup allowing to control hydrodynamic conditions and to monitor biofilm development in real time using microscope imaging. We also conducted a transcriptomic analysis to detect a potential physiological response to hydrodynamics. We discovered that a threshold value of shear stress determined biofilm settlement, with sub-piconewton forces sufficient to prevent biofilm initiation. As a consequence, distinct hydrodynamic conditions, which set spatial distribution of shear stress, promoted distinct colonization patterns with consequences on the growth mode. However, no direct impact of mechanical forces on biofilm growth rate was observed. Consistently, no mechanosensing gene emerged from our differential transcriptomic analysis comparing distinct hydrodynamic conditions. Instead, we found that hydrodynamic molecular transport crucially impacts biofilm growth by controlling oxygen availability. Our results shed light on biofilm response to hydrodynamics and open new avenues to achieve informed design of fluidic setups for investigating, engineering or fighting adherent communities.
Conflict of interest statement
Figures



References
-
- Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu Rev Microbiol. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705 - DOI - PubMed
-
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. Epub 2004/03/26. doi: 10.1038/nrmicro821 - DOI - PubMed
-
- Kolter R, Greenberg EP. Microbial sciences: the superficial life of microbes. Nature. 2006;441(7091):300–2. doi: 10.1038/441300a - DOI - PubMed
-
- Cunliffe M, Murrell JC. The sea-surface microlayer is a gelatinous biofilm. Isme Journal. 2009;3(9):1001–3. doi: 10.1038/ismej.2009.69 - DOI - PubMed
-
- Rusconi R, Garren M, Stocker R. Microfluidics expanding the frontiers of microbial ecology. Annu Rev Biophys. 2014;43:65–91.022916. doi: 10.1146/annurev-biophys-051013-022916 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

