Exercise in vivo marks human myotubes in vitro: Training-induced increase in lipid metabolism
- PMID: 28403174
- PMCID: PMC5389842
- DOI: 10.1371/journal.pone.0175441
Exercise in vivo marks human myotubes in vitro: Training-induced increase in lipid metabolism
Abstract
Background and aims: Physical activity has preventive as well as therapeutic benefits for overweight subjects. In this study we aimed to examine effects of in vivo exercise on in vitro metabolic adaptations by studying energy metabolism in cultured myotubes isolated from biopsies taken before and after 12 weeks of extensive endurance and strength training, from healthy sedentary normal weight and overweight men.
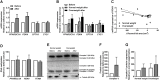
Methods: Healthy sedentary men, aged 40-62 years, with normal weight (body mass index (BMI) < 25 kg/m2) or overweight (BMI ≥ 25 kg/m2) were included. Fatty acid and glucose metabolism were studied in myotubes using [14C]oleic acid and [14C]glucose, respectively. Gene and protein expressions, as well as DNA methylation were measured for selected genes.
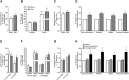
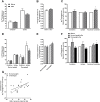
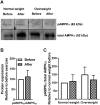
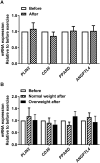
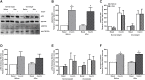
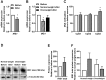
Results: The 12-week training intervention improved endurance, strength and insulin sensitivity in vivo, and reduced the participants' body weight. Biopsy-derived cultured human myotubes after exercise showed increased total cellular oleic acid uptake (30%), oxidation (46%) and lipid accumulation (34%), as well as increased fractional glucose oxidation (14%) compared to cultures established prior to exercise. Most of these exercise-induced increases were significant in the overweight group, whereas the normal weight group showed no change in oleic acid or glucose metabolism.
Conclusions: 12 weeks of combined endurance and strength training promoted increased lipid and glucose metabolism in biopsy-derived cultured human myotubes, showing that training in vivo are able to induce changes in human myotubes that are discernible in vitro.
Conflict of interest statement
Figures
References
-
- Ginter E, Simko V. Type 2 diabetes mellitus, pandemic in 21st century Diabetes: Springer; 2013. p. 42–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

