Binding of interferon reduces the force of unfolding for interferon receptor 1
- PMID: 28403186
- PMCID: PMC5389645
- DOI: 10.1371/journal.pone.0175413
Binding of interferon reduces the force of unfolding for interferon receptor 1
Abstract
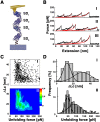
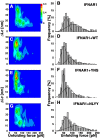
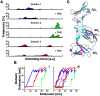
Differential signaling of the type I interferon receptor (IFNAR) has been correlated with the ability of its subunit, IFNAR1, to differentially recognize a large spectrum of different ligands, which involves intricate conformational re-arrangements of multiple interacting domains. To shed light onto the structural determinants governing ligand recognition, we compared the force-induced unfolding of the IFNAR1 ectodomain when bound to interferon and when free, using the atomic force microscope and steered molecular dynamics simulations. Unexpectedly, we find that IFNAR1 is easier to mechanically unfold when bound to interferon than when free. Analysis of the structures indicated that the origin of the reduction in unfolding forces is a conformational change in IFNAR1 induced by ligand binding.
Conflict of interest statement
Figures
Similar articles
-
Ligand binding induces a conformational change in ifnar1 that is propagated to its membrane-proximal domain.J Mol Biol. 2008 Mar 28;377(3):725-39. doi: 10.1016/j.jmb.2008.01.017. Epub 2008 Jan 16. J Mol Biol. 2008. PMID: 18294654
-
The EM structure of a type I interferon-receptor complex reveals a novel mechanism for cytokine signaling.J Mol Biol. 2008 Mar 28;377(3):715-24. doi: 10.1016/j.jmb.2007.12.005. Epub 2007 Dec 8. J Mol Biol. 2008. PMID: 18252254 Free PMC article.
-
Functional cartography of the ectodomain of the type I interferon receptor subunit ifnar1.J Mol Biol. 2005 Jul 15;350(3):476-88. doi: 10.1016/j.jmb.2005.05.008. J Mol Biol. 2005. PMID: 15946680
-
Structural and dynamic determinants of type I interferon receptor assembly and their functional interpretation.Immunol Rev. 2012 Nov;250(1):317-34. doi: 10.1111/imr.12001. Immunol Rev. 2012. PMID: 23046138 Free PMC article. Review.
-
Force spectroscopy studies on protein-ligand interactions: a single protein mechanics perspective.FEBS Lett. 2014 Oct 1;588(19):3613-20. doi: 10.1016/j.febslet.2014.04.009. Epub 2014 Apr 18. FEBS Lett. 2014. PMID: 24747422 Review.
Cited by
-
A Glycoengineered Interferon-β Mutein (R27T) Generates Prolonged Signaling by an Altered Receptor-Binding Kinetics.Front Pharmacol. 2019 Jan 24;9:1568. doi: 10.3389/fphar.2018.01568. eCollection 2018. Front Pharmacol. 2019. PMID: 30733680 Free PMC article.
-
Model systems for optical trapping: the physical basis and biological applications.Biophys Rev. 2021 Jul 27;13(4):515-529. doi: 10.1007/s12551-021-00823-8. eCollection 2021 Aug. Biophys Rev. 2021. PMID: 34471436 Free PMC article. Review.
-
Interferon Receptor Trafficking and Signaling: Journey to the Cross Roads.Front Immunol. 2021 Jan 20;11:615603. doi: 10.3389/fimmu.2020.615603. eCollection 2020. Front Immunol. 2021. PMID: 33552080 Free PMC article. Review.
-
Molecular analysis of the type III interferon complex and its applications in protein engineering.Biophys J. 2023 Nov 7;122(21):4254-4263. doi: 10.1016/j.bpj.2023.09.021. Epub 2023 Oct 4. Biophys J. 2023. PMID: 37794680 Free PMC article.
References
-
- Deonarain R, Chan DC, Platanias LC, Fish EN. Interferon-alpha/beta-receptor interactions: a complex story unfolding. Curr Pharm Des. 2002;8(24):2131–7. - PubMed
-
- Uze G, Schreiber G, Piehler J, Pellegrini S. The receptor of the type I interferon family. Curr Top Microbiol Immunol. 2007;316:71–95. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

