Binding of interferon reduces the force of unfolding for interferon receptor 1
- PMID: 28403186
- PMCID: PMC5389645
- DOI: 10.1371/journal.pone.0175413
Binding of interferon reduces the force of unfolding for interferon receptor 1
Abstract
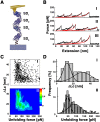
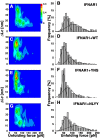
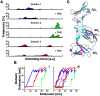
Differential signaling of the type I interferon receptor (IFNAR) has been correlated with the ability of its subunit, IFNAR1, to differentially recognize a large spectrum of different ligands, which involves intricate conformational re-arrangements of multiple interacting domains. To shed light onto the structural determinants governing ligand recognition, we compared the force-induced unfolding of the IFNAR1 ectodomain when bound to interferon and when free, using the atomic force microscope and steered molecular dynamics simulations. Unexpectedly, we find that IFNAR1 is easier to mechanically unfold when bound to interferon than when free. Analysis of the structures indicated that the origin of the reduction in unfolding forces is a conformational change in IFNAR1 induced by ligand binding.
Conflict of interest statement
Figures
References
-
- Deonarain R, Chan DC, Platanias LC, Fish EN. Interferon-alpha/beta-receptor interactions: a complex story unfolding. Curr Pharm Des. 2002;8(24):2131–7. - PubMed
-
- Uze G, Schreiber G, Piehler J, Pellegrini S. The receptor of the type I interferon family. Curr Top Microbiol Immunol. 2007;316:71–95. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

