A novel Meloidogyne graminicola effector, MgGPP, is secreted into host cells and undergoes glycosylation in concert with proteolysis to suppress plant defenses and promote parasitism
- PMID: 28403192
- PMCID: PMC5402989
- DOI: 10.1371/journal.ppat.1006301
A novel Meloidogyne graminicola effector, MgGPP, is secreted into host cells and undergoes glycosylation in concert with proteolysis to suppress plant defenses and promote parasitism
Abstract
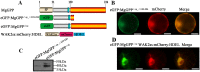
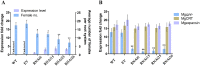
Plant pathogen effectors can recruit the host post-translational machinery to mediate their post-translational modification (PTM) and regulate their activity to facilitate parasitism, but few studies have focused on this phenomenon in the field of plant-parasitic nematodes. In this study, we show that the plant-parasitic nematode Meloidogyne graminicola has evolved a novel effector, MgGPP, that is exclusively expressed within the nematode subventral esophageal gland cells and up-regulated in the early parasitic stage of M. graminicola. The effector MgGPP plays a role in nematode parasitism. Transgenic rice lines expressing MgGPP become significantly more susceptible to M. graminicola infection than wild-type control plants, and conversely, in planta, the silencing of MgGPP through RNAi technology substantially increases the resistance of rice to M. graminicola. Significantly, we show that MgGPP is secreted into host plants and targeted to the ER, where the N-glycosylation and C-terminal proteolysis of MgGPP occur. C-terminal proteolysis promotes MgGPP to leave the ER, after which it is transported to the nucleus. In addition, N-glycosylation of MgGPP is required for suppressing the host response. The research data provide an intriguing example of in planta glycosylation in concert with proteolysis of a pathogen effector, which depict a novel mechanism by which parasitic nematodes could subjugate plant immunity and promote parasitism and may present a promising target for developing new strategies against nematode infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures








Similar articles
-
Rice susceptibility to root-knot nematodes is enhanced by the Meloidogyne incognita MSP18 effector gene.Planta. 2019 Oct;250(4):1215-1227. doi: 10.1007/s00425-019-03205-3. Epub 2019 Jun 19. Planta. 2019. PMID: 31218413
-
The effector MgCRT1 of Meloidogyne graminicola targets the pathogenesis-related (PR) protein OsPR1#101 to facilitate nematodes parasitism in rice.Pest Manag Sci. 2025 Aug;81(8):4705-4713. doi: 10.1002/ps.8831. Epub 2025 Apr 18. Pest Manag Sci. 2025. PMID: 40247744
-
The Meloidogyne graminicola effector Mg16820 is secreted in the apoplast and cytoplasm to suppress plant host defense responses.Mol Plant Pathol. 2018 Nov;19(11):2416-2430. doi: 10.1111/mpp.12719. Epub 2018 Sep 28. Mol Plant Pathol. 2018. PMID: 30011122 Free PMC article.
-
Meloidogyne graminicola: a major threat to rice agriculture.Mol Plant Pathol. 2017 Jan;18(1):3-15. doi: 10.1111/mpp.12394. Epub 2016 Jul 1. Mol Plant Pathol. 2017. PMID: 26950515 Free PMC article. Review.
-
Manipulation of plant cells by cyst and root-knot nematode effectors.Mol Plant Microbe Interact. 2013 Jan;26(1):9-16. doi: 10.1094/MPMI-05-12-0106-FI. Mol Plant Microbe Interact. 2013. PMID: 22809272 Review.
Cited by
-
MiMIF-2 Effector of Meloidogyne incognita Exhibited Enzyme Activities and Potential Roles in Plant Salicylic Acid Synthesis.Int J Mol Sci. 2020 May 15;21(10):3507. doi: 10.3390/ijms21103507. Int J Mol Sci. 2020. PMID: 32429304 Free PMC article.
-
A Meloidogyne graminicola Pectate Lyase Is Involved in Virulence and Activation of Host Defense Responses.Front Plant Sci. 2021 Mar 22;12:651627. doi: 10.3389/fpls.2021.651627. eCollection 2021. Front Plant Sci. 2021. PMID: 33868351 Free PMC article.
-
Transcriptome Analysis of Meloidogyne javanica and the Role of a C-Type Lectin in Parasitism.Plants (Basel). 2024 Mar 4;13(5):730. doi: 10.3390/plants13050730. Plants (Basel). 2024. PMID: 38475576 Free PMC article.
-
A Bursaphelenchus xylophilus effector BxICD1 inducing plant cell death, concurrently contributes to nematode virulence and migration.Front Plant Sci. 2024 Feb 28;15:1357141. doi: 10.3389/fpls.2024.1357141. eCollection 2024. Front Plant Sci. 2024. PMID: 38481400 Free PMC article.
-
The key molecular pattern BxCDP1 of Bursaphelenchus xylophilus induces plant immunity and enhances plant defense response via two small peptide regions.Front Plant Sci. 2022 Aug 3;13:937473. doi: 10.3389/fpls.2022.937473. eCollection 2022. Front Plant Sci. 2022. PMID: 35991456 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

