Synthesis and Evaluation of 2-Naphthaleno trans-Stilbenes and Cyanostilbenes as Anticancer Agents
- PMID: 28403783
- PMCID: PMC7584397
- DOI: 10.2174/1871521409666170412115703
Synthesis and Evaluation of 2-Naphthaleno trans-Stilbenes and Cyanostilbenes as Anticancer Agents
Abstract
Background: Naphthalene is a good structural replacement for the isovanillin moiety (i.e. the 3- hydroxy-4-methoxyphenyl unit) in the combretastatin A-4 molecule, a natural product structurally related to resveratrol, which consistently led to the generation of highly cytotoxic naphthalene analogues when combined with a 3,4,5-trimethoxyphenyl or related aromatic system. Also, the naphthalene ring system is present in many current drug molecules that are utilized for anti-tumor, anti-arrhythmia and antioxidant therapy.
Objective: In our continuing quest to improve the potencies of naturally occurring anti-cancer molecules through chemical modification, we have now synthesized a small library of 2-naphthaleno trans- stilbenes and cyanostilbenes that are structurally related to both resveratrol and DMU-212, and have evaluated these novel analogs against a panel of 54 human tumor cell lines.
Method: A series of 2-naphthaleno-containing trans-stilbenes 3a-3h (Scheme 1) were synthesized by Wittig reaction of a variety of aromatic substituted benzyl-triphenylphosphonium bromide reactants with 2- naphthaldehyde using n-BuLi as a base in THF. A second series of 2-naphthaleno trans-cyanostilbenes analogs 5a-5h was synthesized by reaction of 2-naphthaldehyde (2; 1 mmol) with an appropriately substituted 2- phenylacrylonitrile 4a-4h; 1 mmol) in 5% sodium methoxide/methanol. The reaction mixture was stirred at room temperature for 2-3 hours and the reaction allowed to go to completion (TLC monitoring), during which time the desired product precipitated out of the solution as a solid. The resulting precipitate was filtered off, washed with water and dried to yield the desired compound in yields ranging from 70-95% (Scheme 2).
Results: The percentage growth inhibition of 54 human cancer cell lines in a primary NCI screen after exposure to compounds 3a, 3d, 5b and 5c was carried out. The results showed that only compounds 5b and 5c met the criteria for subsequent testing to determine growth inhibition values (GI50) in dose-response studies. At 10-5 M concentration, compounds 5b and 5c exhibited cytotoxic activity against leukemia cell lines HL-60(TB) and SR, lung cancer cell line NCI-H522, colon cancer cell lines COLO 205 and HCT-116, CNS-cancer cell line SF-539, melanoma cell line MDA-MB-435, and breast cancer cell line BT-549. The naphthalene trans-stilbene analogue 3a, exhibited significant growth inhibition against only one cell line, melanoma cell line MDA-MB-435 (96 % growth inhibition). Compound 3d was inactive in the 10-5 M single dose screen.
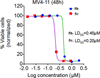
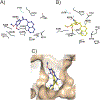
Conclusion: We have synthesized a small set of novel 2-naphthaleno stilbenes and cyanostilbenes and evaluated several of these compounds for their anticancer properties against a panel of 54 human tumor cell lines. The most active analogs, 5b and 5c, showed significantly improved growth inhibition against the human cancer cells in the NCI panel when compared to DMU-212. Of these compounds, analog 5c was found to be the most potent anticancer agent and exhibited significant growth inhibitory effects against COLO 205, CNS SF 539 and melanoma SK-MEL 5 and MDA-MB-435 cell lines with GI50 values ≤ 25 nM. Analog 5b also exhibited GI50 values in the range 25-41 nM against CNS SF 295 and melanoma MDA-MB-435 and UACC-62 cell lines. Compounds 5b and 5c were also cytotoxic towards the MV4-11 leukemia cell line with LD50 value of 450 nM and 200 nM, respectively, and demonstrated >50% inhibition of tubulin polymerization at concentrations below their LD50 values in these cells. In silico docking studies suggest that compounds 5b and 5c bind favorably at the colchicine- binding pocket of the tubulin dimer, indicating that both 5b and 5c may inhibit tubulin polymerization through a mechanism similar to that exhibited by colchicine. Derivative 5c demonstrated more favorable binding based on the docking score and buried surface area, as compared to compound 5b, in agreement with the higher observed potency of 5c against a broader range of tumor cell lines. Based on these results, analog 5c is considered to be a lead compound for further optimization as a clinical candidate for treating a variety of cancers.
Keywords: DMU-212; Resveratrol; cytotoxicity; growth inhibition; naphthalene; tubulin inhibition..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
Figures



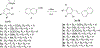
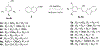
References
-
- Aggarwal BB; Takada Y; Oommen OV From chemoprevention to chemotherapy: common targets and common goals. Exp. Opin. Investig. Drugs, 2004, 13(10), 1327–1338. - PubMed
-
- Baur JA; Pearson KJ; Price NL; Jamieson HA; Lerin C; Kalra A; Prabhu VV; Allard JS; Lopez-Lluch G; Lewis K; Pistell PJ; Poosala S; Becker KG; Boss O; Gwinn D; Wang M; Ramaswamy S; Fishbein KW; Spencer RG; Lakatta EG; Le Couteur D; Shaw RJ; Navas P; Puigserver P; Ingram DK; de Cabo R; Sinclair DA Resveratrol improves health and survival of mice on a high-calorie diet. Nature, 2006, 444(7117), 337–342. - PMC - PubMed
-
- Baur JA; Sinclair DA Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug. Discov, 2006, 5(6), 493–506. - PubMed
-
- Jang M; Cai L; Udeani GO; Slowing KV; Thomas CF; Beecher CW; Fong HH; Farnsworth NR; Kinghorn AD; Mehta RG; Moon RC; Pezzuto JM Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science, 1997, 275(5297), 218–220. - PubMed
-
- Aggarwal BB; Bhardwaj A; Aggarwal RS; Seeram NP; Shishodia S; Takada Y Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res, 2004, 24(5A), 2783–2840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

