Development of a core outcome set for effectiveness trials aimed at optimising prescribing in older adults in care homes
- PMID: 28403876
- PMCID: PMC5389003
- DOI: 10.1186/s13063-017-1915-6
Development of a core outcome set for effectiveness trials aimed at optimising prescribing in older adults in care homes
Abstract
Background: Prescribing medicines for older adults in care homes is known to be sub-optimal. Whilst trials testing interventions to optimise prescribing in this setting have been published, heterogeneity in outcome reporting has hindered comparison of interventions, thus limiting evidence synthesis. The aim of this study was to develop a core outcome set (COS), a list of outcomes which should be measured and reported, as a minimum, for all effectiveness trials involving optimising prescribing in care homes. The COS was developed as part of the Care Homes Independent Pharmacist Prescribing Study (CHIPPS).
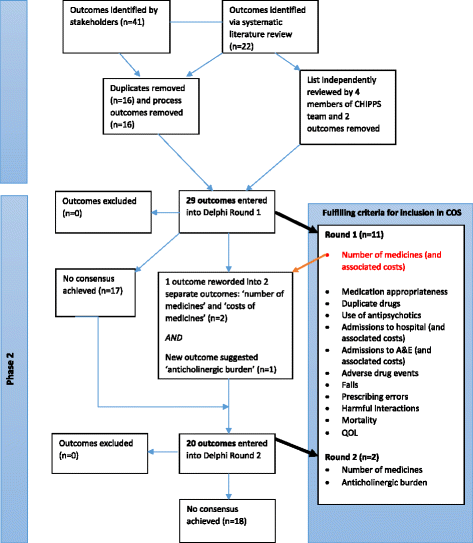
Methods: A long-list of outcomes was identified through a review of published literature and stakeholder input. Outcomes were reviewed and refined prior to entering a two-round online Delphi exercise and then distributed via a web link to the CHIPPS Management Team, a multidisciplinary team including pharmacists, doctors and Patient Public Involvement representatives (amongst others), who comprised the Delphi panel. The Delphi panellists (n = 19) rated the importance of outcomes on a 9-point Likert scale from 1 (not important) to 9 (critically important). Consensus for an outcome being included in the COS was defined as ≥70% participants scoring 7-9 and <15% scoring 1-3. Exclusion was defined as ≥70% scoring 1-3 and <15% 7-9. Individual and group scores were fed back to participants alongside the second questionnaire round, which included outcomes for which no consensus had been achieved.
Results: A long-list of 63 potential outcomes was identified. Refinement of this long-list of outcomes resulted in 29 outcomes, which were included in the Delphi questionnaire (round 1). Following both rounds of the Delphi exercise, 13 outcomes (organised into seven overarching domains: medication appropriateness, adverse drug events, prescribing errors, falls, quality of life, all-cause mortality and admissions to hospital (and associated costs)) met the criteria for inclusion in the final COS.
Conclusions: We have developed a COS for effectiveness trials aimed at optimising prescribing in older adults in care homes using robust methodology. Widespread adoption of this COS will facilitate evidence synthesis between trials. Future work should focus on evaluating appropriate tools for these key outcomes to further reduce heterogeneity in outcome measurement in this context.
Keywords: CHIPPS; COS; Care homes; Consensus; Core outcome set; Delphi technique; Medicines Optimisation; Older adults; Optimising prescribing.
References
-
- Hirsch BR, Califf RM, Cheng SK, Tasneem A, Horton J, Chiswell K, et al. Characteristics of oncology clinical trials: insights from a systematic analysis of ClinicalTrials.gov. JAMA Intern Med. 2013;173:972–9. doi: 10.1001/jamainternmed.2013.627. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous