Mercaptopurine versus placebo to prevent recurrence of Crohn's disease after surgical resection (TOPPIC): a multicentre, double-blind, randomised controlled trial
- PMID: 28404197
- PMCID: PMC6358144
- DOI: 10.1016/S2468-1253(16)30078-4
Mercaptopurine versus placebo to prevent recurrence of Crohn's disease after surgical resection (TOPPIC): a multicentre, double-blind, randomised controlled trial
Abstract
Background: Up to 60% of patients with Crohn's disease need intestinal resection within the first 10 years of diagnosis, and postoperative recurrence is common. We investigated whether mercaptopurine can prevent or delay postoperative clinical recurrence of Crohn's disease.
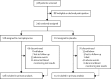
Methods: We did a randomised, placebo-controlled, double-blind trial at 29 UK secondary and tertiary hospitals of patients (aged >16 years in Scotland or >18 years in England and Wales) who had a confirmed diagnosis of Crohn's disease and had undergone intestinal resection. Patients were randomly assigned (1:1) by a computer-generated web-based randomisation system to oral daily mercaptopurine at a dose of 1 mg/kg bodyweight rounded to the nearest 25 mg or placebo; patients with low thiopurine methyltransferase activity received half the normal dose. Patients and their carers and physicians were masked to the treatment allocation. Patients were followed up for 3 years. The primary endpoint was clinical recurrence of Crohn's disease (Crohn's Disease Activity Index >150 plus 100-point increase in score) and the need for anti-inflammatory rescue treatment or primary surgical intervention. Primary and safety analyses were by intention to treat. Subgroup analyses by smoking status, previous thiopurines, previous infliximab or methotrexate, previous surgery, duration of disease, or age at diagnosis were also done. This trial is registered with the International Standard Randomised Controlled Trial Register (ISRCTN89489788) and the European Clinical Trials Database (EudraCT number 2006-005800-15).
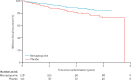
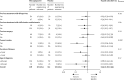
Findings: Between June 6, 2008, and April 23, 2012, 240 patients with Crohn's disease were randomly assigned: 128 to mercaptopurine and 112 to placebo. All patients received at least one dose of study drug, and no randomly assigned patients were excluded from the analysis. 16 (13%) of patients in the mercaptopurine group versus 26 (23%) patients in the placebo group had a clinical recurrence of Crohn's disease and needed anti-inflammatory rescue treatment or primary surgical intervention (adjusted hazard ratio [HR] 0·54, 95% CI 0·27-1·06; p=0·07; unadjusted HR 0·53, 95% CI 0·28-0·99; p=0·046). In a subgroup analysis, three (10%) of 29 smokers in the mercaptopurine group and 12 (46%) of 26 in the placebo group had a clinical recurrence that needed treatment (HR 0·13, 95% CI 0·04-0·46), compared with 13 (13%) of 99 non-smokers in the mercaptopurine group and 14 (16%) of 86 in the placebo group (0·90, 0·42-1·94; pinteraction=0·018). The effect of mercaptopurine did not significantly differ from placebo for any of the other planned subgroup analyses (previous thiopurines, previous infliximab or methotrexate, previous surgery, duration of disease, or age at diagnosis). The incidence and types of adverse events were similar in the mercaptopurine and placebo groups. One patient on placebo died of ischaemic heart disease. Adverse events caused discontinuation of treatment in 39 (30%) of 128 patients in the mercaptopurine group versus 41 (37%) of 112 in the placebo group.
Interpretation: Mercaptopurine is effective in preventing postoperative clinical recurrence of Crohn's disease, but only in patients who are smokers. Thus, in smokers, thiopurine treatment seems to be justified in the postoperative period, although smoking cessation should be strongly encouraged given that smoking increases the risk of recurrence.
Funding: Medical Research Council.
Copyright © 2016 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY license. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
A plea for TDM-based optimisation for treatment of Crohn's disease.Lancet Gastroenterol Hepatol. 2017 Feb;2(2):81. doi: 10.1016/S2468-1253(16)30210-2. Epub 2017 Jan 12. Lancet Gastroenterol Hepatol. 2017. PMID: 28403990 No abstract available.
-
A plea for TDM-based optimisation for treatment of Crohn's disease - Authors' reply.Lancet Gastroenterol Hepatol. 2017 Feb;2(2):81-82. doi: 10.1016/S2468-1253(16)30222-9. Epub 2017 Jan 12. Lancet Gastroenterol Hepatol. 2017. PMID: 28403991 No abstract available.
-
Postoperative thiopurines in smokers with Crohn's disease.Lancet Gastroenterol Hepatol. 2016 Dec;1(4):262-263. doi: 10.1016/S2468-1253(16)30115-7. Epub 2016 Nov 10. Lancet Gastroenterol Hepatol. 2016. PMID: 28404188 No abstract available.
References
-
- Vester-Andersen MK, Prosberg MV, Jess T. Disease course and surgery rates in inflammatory bowel disease: a population-based, 7-year follow-up study in the era of immunomodulating therapy. Am J Gastroenterol. 2014;109:705–714. - PubMed
-
- Van Assche G, Rutgeerts P. Medical management of postoperative recurrence in Crohn's disease. Gastroenterol Clin North Am. 2004;33:347–360. - PubMed
-
- Mowat C, Cole A, Windsor A. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

