Paclitaxel inhibits the activity and membrane localization of PKCα and PKCβI/II to elicit a decrease in stimulated calcitonin gene-related peptide release from cultured sensory neurons
- PMID: 28404507
- PMCID: PMC5954985
- DOI: 10.1016/j.mcn.2017.04.001
Paclitaxel inhibits the activity and membrane localization of PKCα and PKCβI/II to elicit a decrease in stimulated calcitonin gene-related peptide release from cultured sensory neurons
Abstract
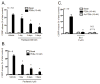
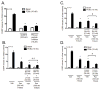
Peripheral neuropathy is a dose-limiting and debilitating side effect of the chemotherapeutic drug, paclitaxel. Consequently, elucidating the mechanisms by which this drug alters sensory neuronal function is essential for the development of successful therapeutics for peripheral neuropathy. We previously demonstrated that chronic treatment with paclitaxel (3-5days) reduces neuropeptide release stimulated by agonists of TRPV1. Because the activity of TRPV1 channels is modulated by conventional and novel PKC isozymes (c/nPKC), we investigated whether c/nPKC mediate the loss of neuropeptide release following chronic treatment with paclitaxel (300nM; 3 and 5days). Release of the neuropeptide, calcitonin gene-related peptide (CGRP), was measured as an index of neuronal sensitivity. Following paclitaxel treatment, cultured dorsal root ganglia sensory neurons were stimulated with a c/nPKC activator, phorbol 12,13-dibutyrate (PDBu), or a TRPV1 agonist, capsaicin, in the absence and presence of selective inhibitors of conventional PKCα and PKCβI/II isozymes (cPKC). Paclitaxel (300nM; 3days and 5days) attenuated both PDBu- and capsaicin-stimulated release in a cPKC-dependent manner. Under basal conditions, there were no changes in the protein expression, phosphorylation or membrane localization of PKC α, βI or βII, however, paclitaxel decreased cPKC activity as indicated by a reduction in the phosphorylation of cPKC substrates. Under stimulatory conditions, paclitaxel attenuated the membrane translocation of phosphorylated PKC α, βI and βII, providing a rationale for the attenuation in PDBu- and capsaicin-stimulated release. Our findings suggest that a decrease in cPKC activity and membrane localization are responsible for the reduction in stimulated peptide release following chronic treatment with paclitaxel in sensory neurons.
Keywords: CGRP; Dorsal root ganglia; PKC; Paclitaxel; Peripheral neuropathy; TRPV1.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



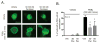

References
-
- Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. Description of a short-term Taxol-induced nociceptive neuropathy in rats. Brain research. 2000;887:239–249. - PubMed
-
- Barber LA, Vasko MR. Activation of protein kinase C augments peptide release from rat sensory neurons. Journal of neurochemistry. 1996;67:72–80. - PubMed
-
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Sudhof TC, Rettig J, Brose N. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21:123–136. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry. 1976;72:248–254. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

