Intravital imaging of the kidney in a rat model of salt-sensitive hypertension
- PMID: 28404591
- PMCID: PMC5582897
- DOI: 10.1152/ajprenal.00466.2016
Intravital imaging of the kidney in a rat model of salt-sensitive hypertension
Abstract
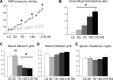
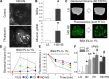
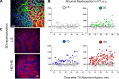
Hypertension is one of the most prevalent diseases worldwide and a major risk factor for renal failure and cardiovascular disease. The role of albuminuria, a common feature of hypertension and robust predictor of cardiorenal disorders, remains incompletely understood. The goal of this study was to investigate the mechanisms leading to albuminuria in the kidney of a rat model of hypertension, the Dahl salt-sensitive (SS) rat. To determine the relative contributions of the glomerulus and proximal tubule (PT) to albuminuria, we applied intravital two-photon-based imaging to investigate the complex renal physiological changes that occur during salt-induced hypertension. Following a high-salt diet, SS rats exhibited elevated blood pressure, increased glomerular sieving of albumin (GSCalb = 0.0686), relative permeability to albumin (+Δ16%), and impaired volume hemodynamics (-Δ14%). Serum albumin but not serum globulins or creatinine concentration was decreased (-0.54 g/dl), which was concomitant with increased filtration of albumin (3.7 vs. 0.8 g/day normal diet). Pathologically, hypertensive animals had significant tubular damage, as indicated by increased prevalence of granular casts, expansion and necrosis of PT epithelial cells (+Δ2.20 score/image), progressive augmentation of red blood cell velocity (+Δ269 µm/s) and micro vessel diameter (+Δ4.3 µm), and increased vascular injury (+Δ0.61 leakage/image). Therefore, development of salt-induced hypertension can be triggered by fast and progressive pathogenic remodeling of PT epithelia, which can be associated with changes in albumin handling. Collectively, these results indicate that both the glomerulus and the PT contribute to albuminuria, and dual treatment of glomerular filtration and albumin reabsorption may represent an effective treatment of salt-sensitive hypertension.
Keywords: albuminuria; chronic kidney disease; glomerulus; proximal tubule.
Copyright © 2017 the American Physiological Society.
Figures



References
-
- Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, Changizi-Ashtiyani S, Bacallao RL, Molitoris BA, Sutton TA. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Renal Physiol 300: F721–F733, 2011. doi: 10.1152/ajprenal.00546.2010. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

