Proteomics Analysis Identifies Orthologs of Human Chitinase-Like Proteins as Inducers of Tube Morphogenesis Defects in Drosophila melanogaster
- PMID: 28404605
- PMCID: PMC5499198
- DOI: 10.1534/genetics.116.199323
Proteomics Analysis Identifies Orthologs of Human Chitinase-Like Proteins as Inducers of Tube Morphogenesis Defects in Drosophila melanogaster
Abstract
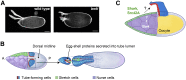
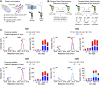
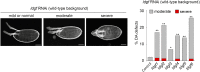
Elevated levels of human chitinase-like proteins (CLPs) are associated with numerous chronic inflammatory diseases and several cancers, often correlating with poor prognosis. Nevertheless, there is scant knowledge of their function. The CLPs normally mediate immune responses and wound healing and, when upregulated, they can promote disease progression by remodeling tissue, activating signaling cascades, stimulating proliferation and migration, and by regulating adhesion. We identified Imaginal disc growth factors (Idgfs), orthologs of human CLPs CHI3L1, CHI3L2, and OVGP1, in a proteomics analysis designed to discover factors that regulate tube morphogenesis in a Drosophila melanogaster model of tube formation. We implemented a novel approach that uses magnetic beads to isolate a small population of specialized ovarian cells, cells that nonautonomously regulate morphogenesis of epithelial tubes that form and secrete eggshell structures called dorsal appendages (DAs). Differential mass spectrometry analysis of these cells detected elevated levels of four of the six Idgf family members (Idgf1, Idgf2, Idgf4, and Idgf6) in flies mutant for bullwinkle (bwk), which encodes a transcription factor and is a known regulator of DA-tube morphogenesis. We show that, during oogenesis, dysregulation of Idgfs (either gain or loss of function) disrupts the formation of the DA tubes. Previous studies demonstrate roles for Drosophila Idgfs in innate immunity, wound healing, and cell proliferation and motility in cell culture. Here, we identify a novel role for Idgfs in both normal and aberrant tubulogenesis processes.
Keywords: chitinase-like proteins; dorsal appendage; imaginal disc growth factors; morphogenesis; proteomics.
Copyright © 2017 by the Genetics Society of America.
Figures



Similar articles
-
Imaginal disk growth factors are Drosophila chitinase-like proteins with roles in morphogenesis and CO2 response.Genetics. 2023 Feb 9;223(2):iyac185. doi: 10.1093/genetics/iyac185. Genetics. 2023. PMID: 36576887 Free PMC article.
-
Detecting New Allies: Modifier Screen Identifies a Genetic Interaction Between Imaginal disc growth factor 3 and combover, a Rho-kinase Substrate, During Dorsal Appendage Tube Formation in Drosophila.G3 (Bethesda). 2020 Oct 5;10(10):3585-3599. doi: 10.1534/g3.120.401476. G3 (Bethesda). 2020. PMID: 32855169 Free PMC article.
-
Chitinases and Imaginal disc growth factors organize the extracellular matrix formation at barrier tissues in insects.Sci Rep. 2016 Feb 3;6:18340. doi: 10.1038/srep18340. Sci Rep. 2016. PMID: 26838602 Free PMC article.
-
Growth factors controlling imaginal disc growth in Drosophila.Novartis Found Symp. 2001;237:182-94; discussion 194-202. Novartis Found Symp. 2001. PMID: 11444043 Review.
-
The missing link: implementation of morphogenetic growth control on the cellular and molecular level.Curr Opin Genet Dev. 2011 Dec;21(6):690-5. doi: 10.1016/j.gde.2011.09.002. Epub 2011 Sep 28. Curr Opin Genet Dev. 2011. PMID: 21959321 Review.
Cited by
-
Imaginal disk growth factors are Drosophila chitinase-like proteins with roles in morphogenesis and CO2 response.Genetics. 2023 Feb 9;223(2):iyac185. doi: 10.1093/genetics/iyac185. Genetics. 2023. PMID: 36576887 Free PMC article.
-
A framework for quality control in quantitative proteomics.bioRxiv [Preprint]. 2024 Aug 11:2024.04.12.589318. doi: 10.1101/2024.04.12.589318. bioRxiv. 2024. Update in: J Proteome Res. 2024 Oct 4;23(10):4392-4408. doi: 10.1021/acs.jproteome.4c00363. PMID: 38645098 Free PMC article. Updated. Preprint.
-
Detecting New Allies: Modifier Screen Identifies a Genetic Interaction Between Imaginal disc growth factor 3 and combover, a Rho-kinase Substrate, During Dorsal Appendage Tube Formation in Drosophila.G3 (Bethesda). 2020 Oct 5;10(10):3585-3599. doi: 10.1534/g3.120.401476. G3 (Bethesda). 2020. PMID: 32855169 Free PMC article.
-
The indispensable contribution of s38 protein to ovarian-eggshell morphogenesis in Drosophila melanogaster.Sci Rep. 2018 Oct 31;8(1):16103. doi: 10.1038/s41598-018-34532-2. Sci Rep. 2018. PMID: 30382186 Free PMC article.
-
Neural Ganglia Transcriptome and Peptidome Associated with Sexual Maturation in Female Pacific Abalone (Haliotis discus hannai).Genes (Basel). 2019 Apr 2;10(4):268. doi: 10.3390/genes10040268. Genes (Basel). 2019. PMID: 30987054 Free PMC article.
References
-
- Bourbon H. M., Gonzy-Treboul G., Peronnet F., Alin M. F., Ardourel C., et al. , 2002. A P-insertion screen identifying novel X-linked essential genes in Drosophila. Mech. Dev. 110: 71–83. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

