Myeloperoxidase Mediates Postischemic Arrhythmogenic Ventricular Remodeling
- PMID: 28404615
- PMCID: PMC5482785
- DOI: 10.1161/CIRCRESAHA.117.310870
Myeloperoxidase Mediates Postischemic Arrhythmogenic Ventricular Remodeling
Abstract
Rationale: Ventricular arrhythmias remain the leading cause of death in patients suffering myocardial ischemia. Myeloperoxidase, a heme enzyme released by polymorphonuclear neutrophils, accumulates within ischemic myocardium and has been linked to adverse left ventricular remodeling.
Objective: To reveal the role of myeloperoxidase for the development of ventricular arrhythmias.
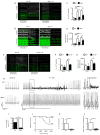
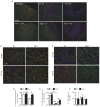
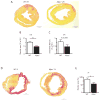
Methods and results: In different murine models of myocardial ischemia, myeloperoxidase deficiency profoundly decreased vulnerability for ventricular tachycardia on programmed right ventricular and burst stimulation and spontaneously as assessed by ECG telemetry after isoproterenol injection. Experiments using CD11b/CD18 integrin-deficient (CD11b-/-) mice and intravenous myeloperoxidase infusion revealed that neutrophil infiltration is a prerequisite for myocardial myeloperoxidase accumulation. Ventricles from myeloperoxidase-deficient (Mpo-/-) mice showed less pronounced slowing and decreased heterogeneity of electric conduction in the peri-infarct zone than wild-type mice. Expression of the redox-sensitive gap junctional protein Cx43 (Connexin 43) was reduced in the peri-infarct area of wild-type compared with Mpo-/- mice. In isolated wild-type cardiomyocytes, Cx43 protein content decreased on myeloperoxidase/H2O2 incubation. Mapping of induced pluripotent stem cell-derived cardiomyocyte networks and in vivo investigations linked Cx43 breakdown to myeloperoxidase-dependent activation of matrix metalloproteinase 7. Moreover, Mpo-/- mice showed decreased ventricular postischemic fibrosis reflecting reduced accumulation of myofibroblasts. Ex vivo, myeloperoxidase was demonstrated to induce fibroblast-to-myofibroblast transdifferentiation by activation of p38 mitogen-activated protein kinases resulting in upregulated collagen generation. In support of our experimental findings, baseline myeloperoxidase plasma levels were independently associated with a history of ventricular arrhythmias, sudden cardiac death, or implantable cardioverter-defibrillator implantation in a cohort of 2622 stable patients with an ejection fraction >35% undergoing elective diagnostic cardiac evaluation.
Conclusions: Myeloperoxidase emerges as a crucial mediator of postischemic myocardial remodeling and may evolve as a novel pharmacological target for secondary disease prevention after myocardial ischemia.
Keywords: Connexin 43; fibrosis; infarction; inflammation; myocardial ischemia; tachycardia.
© 2017 American Heart Association, Inc.
Figures




Comment in
-
Immune Modulation of Cardiac Arrhythmias.Circ Res. 2017 Jun 23;121(1):11-12. doi: 10.1161/CIRCRESAHA.117.311214. Circ Res. 2017. PMID: 28642323 Free PMC article. No abstract available.
References
-
- Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Circulation. 2006;114 - PubMed
-
- Lau D, Baldus S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol Ther. 2006;111:16–26. - PubMed
-
- Abu-Soud HM, Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem. 2000;275:37524–32. - PubMed
-
- Vasilyev N, Williams T, Brennan M-L, Unzek S, Zhou X, Heinecke JW, Spitz DR, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 2005;112:2812–20. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

