Comparison of ePlex Respiratory Pathogen Panel with Laboratory-Developed Real-Time PCR Assays for Detection of Respiratory Pathogens
- PMID: 28404682
- PMCID: PMC5442551
- DOI: 10.1128/JCM.00221-17
Comparison of ePlex Respiratory Pathogen Panel with Laboratory-Developed Real-Time PCR Assays for Detection of Respiratory Pathogens
Abstract
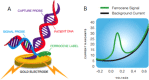
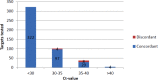
Infections of the respiratory tract can be caused by a diversity of pathogens, both viral and bacterial. Rapid microbiological diagnosis ensures appropriate antimicrobial therapy as well as effective implementation of isolation precautions. The ePlex respiratory pathogen panel (RP panel) is a novel molecular biology-based assay, developed by GenMark Diagnostics, Inc. (Carlsbad, CA), to be performed within a single cartridge for the diagnosis of 25 respiratory pathogens (viral and bacterial). The objective of this study was to compare the performance of the RP panel with those of laboratory-developed real-time PCR assays, using a variety of previously collected clinical respiratory specimens. A total of 343 clinical specimens as well as 29 external quality assessment (EQA) specimens and 2 different Middle East respiratory syndrome coronavirus isolates have been assessed in this study. The RP panel showed an agreement of 97.4% with the real-time PCR assay regarding 464 pathogens found in the clinical specimens. All pathogens present in clinical samples and EQA samples with a threshold cycle (CT ) value of <30 were detected correctly using the RP panel. The RP panel detected 17 additional pathogens, 7 of which could be confirmed by discrepant testing. In conclusion, this study shows excellent performance of the RP panel in comparison to real-time PCR assays for the detection of respiratory pathogens. The ePlex system provided a large amount of useful diagnostic data within a short time frame, with minimal hands-on time, and can therefore potentially be used for rapid diagnostic sample-to-answer testing, in either a laboratory or a decentralized setting.
Keywords: influenza; point-of-care testing; rapid diagnostics; respiratory pathogens; sample-to-answer.
Copyright © 2017 Nijhuis et al.
Figures

References
-
- Gaunt ER, Hardie A, Claas EC, Simmonds P, Templeton KE. 2010. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol 48:2940–2947. doi: 10.1128/JCM.00636-10. - DOI - PMC - PubMed
-
- Templeton KE, Scheltinga SA, Beersma MF, Kroes AC, Claas EC. 2004. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza a and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol 42:1564–1569. doi: 10.1128/JCM.42.4.1564-1569.2004. - DOI - PMC - PubMed
-
- Templeton KE, Scheltinga SA, Graffelman AW, Van Schie JM, Crielaard JW, Sillekens P, Van Den Broek PJ, Goossens H, Beersma MF, Claas EC. 2003. Comparison and evaluation of real-time PCR, real-time nucleic acid sequence-based amplification, conventional PCR, and serology for diagnosis of Mycoplasma pneumoniae. J Clin Microbiol 41:4366–4371. doi: 10.1128/JCM.41.9.4366-4371.2003. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

