Interaction of compass sensing and object-motion detection in the locust central complex
- PMID: 28404828
- PMCID: PMC5511872
- DOI: 10.1152/jn.00927.2016
Interaction of compass sensing and object-motion detection in the locust central complex
Abstract
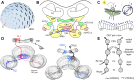
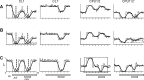
Goal-directed behavior is often complicated by unpredictable events, such as the appearance of a predator during directed locomotion. This situation requires adaptive responses like evasive maneuvers followed by subsequent reorientation and course correction. Here we study the possible neural underpinnings of such a situation in an insect, the desert locust. As in other insects, its sense of spatial orientation strongly relies on the central complex, a group of midline brain neuropils. The central complex houses sky compass cells that signal the polarization plane of skylight and thus indicate the animal's steering direction relative to the sun. Most of these cells additionally respond to small moving objects that drive fast sensory-motor circuits for escape. Here we investigate how the presentation of a moving object influences activity of the neurons during compass signaling. Cells responded in one of two ways: in some neurons, responses to the moving object were simply added to the compass response that had adapted during continuous stimulation by stationary polarized light. By contrast, other neurons disadapted, i.e., regained their full compass response to polarized light, when a moving object was presented. We propose that the latter case could help to prepare for reorientation of the animal after escape. A neuronal network based on central-complex architecture can explain both responses by slight changes in the dynamics and amplitudes of adaptation to polarized light in CL columnar input neurons of the system.NEW & NOTEWORTHY Neurons of the central complex in several insects signal compass directions through sensitivity to the sky polarization pattern. In locusts, these neurons also respond to moving objects. We show here that during polarized-light presentation, responses to moving objects override their compass signaling or restore adapted inhibitory as well as excitatory compass responses. A network model is presented to explain the variations of these responses that likely serve to redirect flight or walking following evasive maneuvers.
Keywords: central complex; context dependency; desert locust; escape behavior; gain modulation; goal conflict; insect brain; neural modeling; sky compass; spatial orientation.
Copyright © 2017 the American Physiological Society.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

