A dose-dependent plasma signature of the safety and immunogenicity of the rVSV-Ebola vaccine in Europe and Africa
- PMID: 28404856
- PMCID: PMC6379059
- DOI: 10.1126/scitranslmed.aaj1701
A dose-dependent plasma signature of the safety and immunogenicity of the rVSV-Ebola vaccine in Europe and Africa
Erratum in
-
Erratum for the Research Article: "A dose-dependent plasma signature of the safety and immunogenicity of the rVSV-Ebola vaccine in Europe and Africa" by A. Huttner, C. Combescure, S. Grillet, M. C. Haks, E. Quinten, C. Modoux, S. T. Agnandji, J. Brosnahan, J.-A. Dayer, A. M. Harandi, L. Kaiser, D. Medaglini, T. Monath, VEBCON and VSV-EBOVAC Consortia, P. Roux-Lombard, P. G. Kremsner, T. H. M. Ottenhoff, C.-A. Siegrist.Sci Transl Med. 2019 Aug 21;11(506):eaaz0296. doi: 10.1126/scitranslmed.aaz0296. Sci Transl Med. 2019. PMID: 31434753 No abstract available.
Abstract
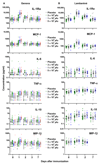
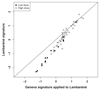
The 2014-2015 Ebola epidemic affected several African countries, claiming more than 11,000 lives and leaving thousands with ongoing sequelae. Safe and effective vaccines could prevent or limit future outbreaks. The recombinant vesicular stomatitis virus-vectored Zaire Ebola (rVSV-ZEBOV) vaccine has shown marked immunogenicity and efficacy in humans but is reactogenic at higher doses. To understand its effects, we examined plasma samples from 115 healthy volunteers from Geneva who received low-dose (LD) or high-dose (HD) vaccine or placebo. Fifteen plasma chemokines/cytokines were assessed at baseline and on days 1, 2 to 3, and 7 after injection. Significant increases in monocyte-mediated MCP-1/CCL2, MIP-1β/CCL4, IL-6, TNF-α, IL-1Ra, and IL-10 occurred on day 1. A signature explaining 68% of cytokine/chemokine vaccine-response variability was identified. Its score was higher in HD versus LD vaccinees and was associated positively with vaccine viremia and negatively with cytopenia. It was higher in vaccinees with injection-site pain, fever, myalgia, chills, and headache; higher scores reflected increasing severity. In contrast, HD vaccinees who subsequently developed arthritis had lower day 1 scores than other HD vaccinees. Vaccine dose did not influence the signature despite its influence on specific outcomes. The Geneva-derived signature associated strongly (ρ = 0.97) with that of a cohort of 75 vaccinees from a parallel trial in Lambaréné, Gabon. Its score in Geneva HD vaccinees with subsequent arthritis was significantly lower than that in Lambaréné HD vaccinees, none of whom experienced arthritis. This signature, which reveals monocytes' critical role in rVSV-ZEBOV immunogenicity and safety across doses and continents, should prove useful in assessments of other vaccines.
Copyright © 2017, American Association for the Advancement of Science.
Conflict of interest statement
Figures

References
-
- Kanapathipillai R, Henao Restrepo AM, Fast P, Wood D, Dye C, Kieny M-P, Moorthy V. Ebola vaccine—An urgent international priority. N Engl J Med. 2014;371:2249–2251. - PubMed
-
- Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, Carroll MW, Dean NE, Diatta I, Doumbia M, Draguez B, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!) Lancet. 2017;389:505–518. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

