Surpassing light-induced cell damage in vitro with novel cell culture media
- PMID: 28405003
- PMCID: PMC5429800
- DOI: 10.1038/s41598-017-00829-x
Surpassing light-induced cell damage in vitro with novel cell culture media
Abstract
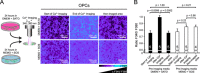
Light is extensively used to study cells in real time (live cell imaging), separate cells using fluorescence activated cell sorting (FACS) and control cellular functions with light sensitive proteins (Optogenetics). However, photo-sensitive molecules inside cells and in standard cell culture media generate toxic by-products that interfere with cellular functions and cell viability when exposed to light. Here we show that primary cells from the rat central nervous system respond differently to photo-toxicity, in that astrocytes and microglia undergo morphological changes, while in developing neurons and oligodendrocyte progenitor cells (OPCs) it induces cellular death. To prevent photo-toxicity and to allow for long-term photo-stimulation without causing cellular damage, we formulated new photo-inert media called MEMO and NEUMO, and an antioxidant rich and serum free supplement called SOS. These new media reduced the detrimental effects caused by light and allowed cells to endure up to twenty times more light exposure without adverse effects, thus bypassing the optical constraints previously limiting experiments.
Conflict of interest statement
Under a licensing agreement between Cambridge Enterprise and Cell Guidance Systems, J.H.S. and R.T.K. are entitled to a share of royalty received by sales of MEMO, NEUMO and SOS, an arrangement being managed by Cambridge Enterprise and Cell Guidance Systems.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

