Therapeutic reduction of ataxin-2 extends lifespan and reduces pathology in TDP-43 mice
- PMID: 28405022
- PMCID: PMC5642042
- DOI: 10.1038/nature22038
Therapeutic reduction of ataxin-2 extends lifespan and reduces pathology in TDP-43 mice
Abstract
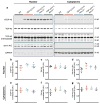
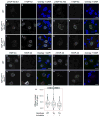
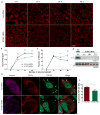
Amyotrophic lateral sclerosis (ALS) is a rapidly progressing neurodegenerative disease that is characterized by motor neuron loss and that leads to paralysis and death 2-5 years after disease onset. Nearly all patients with ALS have aggregates of the RNA-binding protein TDP-43 in their brains and spinal cords, and rare mutations in the gene encoding TDP-43 can cause ALS. There are no effective TDP-43-directed therapies for ALS or related TDP-43 proteinopathies, such as frontotemporal dementia. Antisense oligonucleotides (ASOs) and RNA-interference approaches are emerging as attractive therapeutic strategies in neurological diseases. Indeed, treatment of a rat model of inherited ALS (caused by a mutation in Sod1) with ASOs against Sod1 has been shown to substantially slow disease progression. However, as SOD1 mutations account for only around 2-5% of ALS cases, additional therapeutic strategies are needed. Silencing TDP-43 itself is probably not appropriate, given its critical cellular functions. Here we present a promising alternative therapeutic strategy for ALS that involves targeting ataxin-2. A decrease in ataxin-2 suppresses TDP-43 toxicity in yeast and flies, and intermediate-length polyglutamine expansions in the ataxin-2 gene increase risk of ALS. We used two independent approaches to test whether decreasing ataxin-2 levels could mitigate disease in a mouse model of TDP-43 proteinopathy. First, we crossed ataxin-2 knockout mice with TDP-43 (also known as TARDBP) transgenic mice. The decrease in ataxin-2 reduced aggregation of TDP-43, markedly increased survival and improved motor function. Second, in a more therapeutically applicable approach, we administered ASOs targeting ataxin-2 to the central nervous system of TDP-43 transgenic mice. This single treatment markedly extended survival. Because TDP-43 aggregation is a component of nearly all cases of ALS, targeting ataxin-2 could represent a broadly effective therapeutic strategy.
Conflict of interest statement
P.J.-N., A.S. and F.R. are employed by Ionis Pharmaceuticals, a for-profit company that develops ASO therapies. The other authors declare no competing financial interest.
Figures


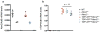




Comment in
-
Neurodegenerative disease: Two-for-one on potential therapies.Nature. 2017 Apr 20;544(7650):302-303. doi: 10.1038/nature21911. Epub 2017 Apr 12. Nature. 2017. PMID: 28405020 No abstract available.
-
Neurodegenerative disorders: Ataxin 2 reduction rescues motor defects.Nat Rev Drug Discov. 2017 Jun;16(6):384-385. doi: 10.1038/nrd.2017.104. Epub 2017 May 19. Nat Rev Drug Discov. 2017. PMID: 28529318 No abstract available.
-
'Forward genetics' and the causes of ALS.Nat Rev Mol Cell Biol. 2019 Feb;20(2):67. doi: 10.1038/s41580-018-0062-6. Nat Rev Mol Cell Biol. 2019. PMID: 30279566 No abstract available.
References
-
- Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. - PubMed
-
- Southwell AL, Skotte NH, Bennett CF, Hayden MR. Antisense oligonucleotide therapeutics for inherited neurodegenerative diseases. Trends Mol Med. 2012;18:634–643. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

