Evaluation of Dehydroepiandrosterone Supplementation on Diminished Ovarian Reserve: A Randomized, Double-Blinded, Placebo-Controlled Study
- PMID: 28405122
- PMCID: PMC5371526
- DOI: 10.1007/s13224-016-0941-8
Evaluation of Dehydroepiandrosterone Supplementation on Diminished Ovarian Reserve: A Randomized, Double-Blinded, Placebo-Controlled Study
Abstract
Introduction: We conducted a randomized, double-blinded, placebo-controlled study, to evaluate the effect of dehydroepiandrosterone (DHEA), on diminished ovarian reserve (DOR).
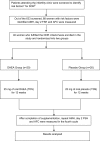
Materials and methods: Twenty patients with DOR received DHEA (oral 25 mg three times a day). Post-supplementation 12 weeks, D2/3 age-specific follicle-stimulating hormone (FSH), anti-mullerian hormone (AMH) levels, and antral follicle count (AFC), were repeated to evaluate response. Spontaneous pregnancy rates and regularization of menstrual cycles were also studied as secondary outcome.
Results: Predominant risk factors were age >35 years (28 %) and poor responders to ovarian stimulation (23 %). There was significant improvement of AMH levels (1.15 ± 1.49 vs. 1.53 ± 1.62) found before and after supplementation in the DHEA group. When the AMH values between DHEA and placebo group were compared, pre- and post-supplementation, no significant difference was found. There was decrease in FSH levels and increase in AFC value post-supplementation in both DHEA and placebo groups which was not statically significant. DHEA supplementation benefited clinically, as evidenced by the improvement in the menstrual abnormality spontaneous conception in two cases each.
Conclusions: A significant improvement in AMH levels pre- and post-supplementation of DHEA was noted. The same was not seen for FSH and AFC values.
Keywords: Anti-mullerian hormone; Antral follicle count; Dehydroepiandrosterone; Diminished ovarian reserve; FSH.
Conflict of interest statement
Conflict of interest
None of the authors have any conflict of interest or financial conflicts. The authors have nothing to disclose.
Research Involving Human Participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants, and institutional ethical clearance was obtained for the study.
Figures
Similar articles
-
Dehydroepiandrosterone supplementation improves predictive markers for diminished ovarian reserve: serum AMH, inhibin B and antral follicle count.Eur J Obstet Gynecol Reprod Biol. 2013 Jul;169(2):257-60. doi: 10.1016/j.ejogrb.2013.04.003. Epub 2013 May 9. Eur J Obstet Gynecol Reprod Biol. 2013. PMID: 23664458 Clinical Trial.
-
A randomized double-blinded placebo-controlled trial on the effect of dehydroepiandrosterone for 16 weeks on ovarian response markers in women with primary ovarian insufficiency.J Clin Endocrinol Metab. 2013 Jan;98(1):380-8. doi: 10.1210/jc.2012-3071. Epub 2012 Nov 8. J Clin Endocrinol Metab. 2013. PMID: 23144466 Clinical Trial.
-
The effect of dehydroepiandrosterone supplementation on ovarian response is associated with androgen receptor in diminished ovarian reserve women.J Ovarian Res. 2017 May 4;10(1):32. doi: 10.1186/s13048-017-0326-3. J Ovarian Res. 2017. PMID: 28472976 Free PMC article. Clinical Trial.
-
A meta-analysis of dehydroepiandrosterone supplementation among women with diminished ovarian reserve undergoing in vitro fertilization or intracytoplasmic sperm injection.Int J Gynaecol Obstet. 2015 Dec;131(3):240-5. doi: 10.1016/j.ijgo.2015.06.028. Epub 2015 Sep 6. Int J Gynaecol Obstet. 2015. PMID: 26421833 Review.
-
The effect of dehydroepiandrosterone (DHEA) supplementation on women with diminished ovarian reserve (DOR) in IVF cycle: Evidence from a meta-analysis.J Gynecol Obstet Hum Reprod. 2017 Jan;46(1):1-7. doi: 10.1016/j.jgyn.2016.01.002. Epub 2016 May 19. J Gynecol Obstet Hum Reprod. 2017. PMID: 28403950 Review.
Cited by
-
The effect of medication on serum anti-müllerian hormone (AMH) levels in women of reproductive age: a meta-analysis.BMC Endocr Disord. 2022 Jun 14;22(1):158. doi: 10.1186/s12902-022-01065-9. BMC Endocr Disord. 2022. PMID: 35698127 Free PMC article.
-
The Relationships Between Serum DHEA-S and AMH Levels in Infertile Women: A Retrospective Cross-Sectional Study.J Clin Med. 2021 Mar 15;10(6):1211. doi: 10.3390/jcm10061211. J Clin Med. 2021. PMID: 33803980 Free PMC article.
-
Efficacy of dehydroepiandrosterone priming in women with poor ovarian response undergoing IVF/ICSI: a meta-analysis.Front Endocrinol (Lausanne). 2023 Jun 9;14:1156280. doi: 10.3389/fendo.2023.1156280. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37361534 Free PMC article.
-
The Role of Androgen Supplementation in Women With Diminished Ovarian Reserve: Time to Randomize, Not Meta-Analyze.Front Endocrinol (Lausanne). 2021 May 17;12:653857. doi: 10.3389/fendo.2021.653857. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34079524 Free PMC article. Review.
-
Androgens (dehydroepiandrosterone or testosterone) for women undergoing assisted reproduction.Cochrane Database Syst Rev. 2024 Jun 5;6(6):CD009749. doi: 10.1002/14651858.CD009749.pub3. Cochrane Database Syst Rev. 2024. PMID: 38837771 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
