Oxidant-induced autophagy and ferritin degradation contribute to epithelial-mesenchymal transition through lysosomal iron
- PMID: 28405169
- PMCID: PMC5378460
- DOI: 10.2147/JIR.S128292
Oxidant-induced autophagy and ferritin degradation contribute to epithelial-mesenchymal transition through lysosomal iron
Abstract
Purpose: Transforming growth factor (TGF)-β1 triggers epithelial-mesenchymal transition (EMT) through autophagy, which is partly driven by reactive oxygen species (ROS). The aim of this study was to determine whether leaking lysosomes and enhanced degradation of H-ferritin could be involved in EMT and whether it could be possible to prevent EMT by iron chelation targeting of the lysosome.
Materials and methods: EMT, H-ferritin, and autophagy were evaluated in TGF-β1-stimulated A549 human lung epithelial cells cultured in vitro using Western blotting, with the additional morphological assessment of EMT. By using immunofluorescence and flow cytometry, lysosomes and ROS were assessed by acridine orange and 6-carboxy-2',7'-dichlorodihydrofluorescein acetate assays, respectively.
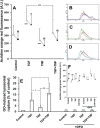
Results: TGF-β1-stimulated cells demonstrated a loss of H-ferritin, which was prevented by the antioxidant N-acetyl-L-cysteine (NAC) and inhibitors of lysosomal degradation. TGF-β1 stimulation generated ROS and autophagosome formation and led to EMT, which was further promoted by the additional ROS-generating cytokine, tumor necrosis factor-α. Lysosomes of TGF-β1-stimulated cells were sensitized to oxidants but also completely protected by lysosomal loading with dextran-bound deferoxamine (DFO). Autophagy and EMT were prevented by NAC, DFO, and inhibitors of autophagy and lysosomal degradation.
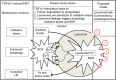
Conclusion: The findings of this study support the role of enhanced autophagic degradation of H-ferritin as a mechanism for increasing the vulnerability of lysosomes to iron-driven oxidant injury that triggers further autophagy during EMT. This study proposes that lysosomal leakage is a novel pathway of TGF-β1-induced EMT that may be prevented by iron-chelating drugs that target the lysosome.
Keywords: A549 cells; COPD; pulmonary disease; pulmonary fibrosis; transforming growth factor; tumor necrosis factor.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures




References
-
- Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L525–L534. - PubMed
-
- Nowrin K, Sohal SS, Peterson G, Patel R, Walters EH. Epithelial-mesenchymal transition as a fundamental underlying pathogenic process in COPD airways: fibrosis, remodeling and cancer. Expert Rev Respir Med. 2014;8(5):547–559. - PubMed
-
- Cannito S, Novo E, di Bonzo LV, Busletta C, Colombatto S, Parola M. Epithelial-mesenchymal transition: from molecular mechanisms, redox regulation to implications in human health and disease. Antioxid Redox Signal. 2010;12(12):1383–1430. - PubMed
-
- Arsalane K, Dubois CM, Muanza T, et al. Transforming growth factor-beta1 is a potent inhibitor of glutathione synthesis in the lung epithelial cell line A549: transcriptional effect on the GSH rate-limiting enzyme gamma-glutamylcysteine synthetase. Am J Respir Cell Mol Biol. 1997;17(5):599–607. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

