Contribution of microreactor technology and flow chemistry to the development of green and sustainable synthesis
- PMID: 28405232
- PMCID: PMC5372749
- DOI: 10.3762/bjoc.13.51
Contribution of microreactor technology and flow chemistry to the development of green and sustainable synthesis
Abstract
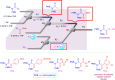
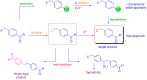
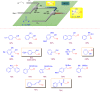
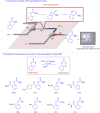
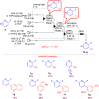
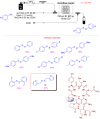
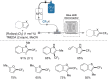
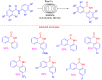
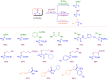
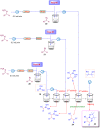
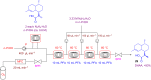
Microreactor technology and flow chemistry could play an important role in the development of green and sustainable synthetic processes. In this review, some recent relevant examples in the field of flash chemistry, catalysis, hazardous chemistry and continuous flow processing are described. Selected examples highlight the role that flow chemistry could play in the near future for a sustainable development.
Keywords: flash chemistry; flow chemistry; green chemistry; microreactor technology; sustainable synthesis.
Figures















References
-
- [May 13;2014 ];Navigant Pike Research Study. Available from: http://www.navigantresearch.com/research/green-chemistry.
-
- Reschetilowski W, editor. Microreactors in Preparative Chemistry. Weinheim: Wiley-VCH; 2013.
-
- Nagaki A, Yoshida J-I. Top Organomet Chem. 2015;57:137–175. doi: 10.1007/3418_2015_154. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
