Novel β-cyclodextrin-eosin conjugates
- PMID: 28405233
- PMCID: PMC5372748
- DOI: 10.3762/bjoc.13.52
Novel β-cyclodextrin-eosin conjugates
Abstract
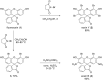
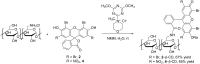
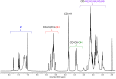
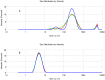
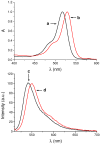
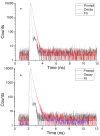
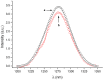
Eosin B (EoB) and eosin Y (EoY), two xanthene dye derivatives with photosensitizing ability were prepared in high purity through an improved synthetic route. The dyes were grafted to a 6-monoamino-β-cyclodextrin scaffold under mild reaction conditions through a stable amide linkage using the coupling agent 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride. The molecular conjugates, well soluble in aqueous medium, were extensively characterized by 1D and 2D NMR spectroscopy and mass spectrometry. Preliminary spectroscopic investigations showed that the β-cyclodextrin-EoY conjugate retains both the fluorescence properties and the capability to photogenerate singlet oxygen of the unbound chromophore. In contrast, the corresponding β-cyclodextrin-EoB conjugate did not show either relevant emission or photosensitizing activity probably due to aggregation in aqueous medium, which precludes any response to light excitation.
Keywords: fluorescence; photodynamic therapy; photosensitizers; singlet oxygen; xanthene; β-cyclodextrins.
Figures

References
-
- Szejtli J. Pure Appl Chem. 2004;76:1825–1845. doi: 10.1351/pac200476101825. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
