Multiple Intravitreal Ranibizumab Injections for Persistant Choroidal Neovascularization Associated with Presumed Ocular Histoplasmosis Syndrome
- PMID: 28405488
- PMCID: PMC5384118
- DOI: 10.4274/tjo.82956
Multiple Intravitreal Ranibizumab Injections for Persistant Choroidal Neovascularization Associated with Presumed Ocular Histoplasmosis Syndrome
Abstract
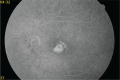
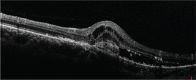
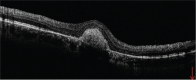
Presumed ocular histoplasmosis syndrome (POHS) is a clinical entity that is characterized by small, round, discrete, macular or mid peripheral atrophic (punched out) chorioretinal lesions (histo spots), peripapillary scarring, choroidal neovascularization (CNV), and the absence of anterior uveitis and vitritis. Diagnosis of this disorder is based upon characteristic clinical findings and a positive histoplasmin skin test or residence in an endemic region for Histoplasma capsulatum. There is no active systemic disease during diagnosis of POHS. Disciform scarring and macular CNV secondary to POHS is a well-known complication which leads to loss of visual acuity or visual disturbance. Without therapy, the visual prognosis in these patients is unfavorable. Submacular surgery, radiation, steroids, photodynamic therapy, and most recently anti-vascular endothelial growth factor therapy are current therapeutic options for this condition. We report a case with persistent CNV secondary to POHS in a middle-aged woman with moderate myopia and the clinical course of treatment with multiple intravitreal ranibizumab (Lucentis®, Novartis) injections.
Keywords: choroidal neovascularization; histoplasmosis; intravitreal injection; ranibizumab.
Conflict of interest statement
Conflict of Interest: No conflict of interest was declared by the authors.
Figures


References
-
- Woods AC, Wahlen HE. The propable role of benign histoplasmosis in the etiology of granulomatous uveitis. Am J Ophthalmol. 1960;49:205–220. - PubMed
-
- Adan A, Navarro M, Casaroli-Marano RP, Ortiz S, Molina JJ. Intravitreal bevacizumab as initial treatment for choroidal neovascularization associated with presumed ocular histoplasmosis syndrome. Graefes Arch Clin Exp Ophthalmol. 2007;245:1873–1875. - PubMed
-
- Tsai JH, Smith RE. Ocular Histoplasmosis. In: Albert DM, Miller JW, Azar DT, Blodi BA, editors. Albert and Jacobiec’s Principles and Practice of Ophthalmolgy (3rd ed) Saunders Elsevier: North America; 2008. pp. 1211–1220.
-
- Trevino R, Salvat R. Preventing reactivation of ocular histoplasmosis: guidance for patients at risk. Optometry. 2006;77:10–16. - PubMed
-
- Tsai CB, Tan CY, Yang PM, Chang KP. Bevacizumab treatment for choroidal neovascularization in a patient with chorioretinopathy resembling presumed ocular histoplasmosis syndrome. Taiwan J Ophthalmol. 2012;2:144–147.
LinkOut - more resources
Full Text Sources
Other Literature Sources
