Combining therapeutic antibodies using basiliximab and etanercept for severe steroid-refractory acute graft-versus-host disease: A multi-center prospective study
- PMID: 28405499
- PMCID: PMC5384382
- DOI: 10.1080/2162402X.2016.1277307
Combining therapeutic antibodies using basiliximab and etanercept for severe steroid-refractory acute graft-versus-host disease: A multi-center prospective study
Abstract
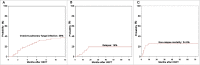
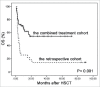
Acute graft versus host disease (aGVHD) remains a major problem after allogeneic hematopoietic stem cell transplantation. Standard frontline therapy for aGVHD involves corticosteroids. However, fewer than half of patients have a lasting complete response. The long-term mortality rate of steroid-refractory aGVHD (SR-aGVHD) remains around 70%. To date, no consensus has been reached regarding the optimal salvage treatment for SR-aGVHD. We performed the first prospective, multi-center clinical trial to assess the efficacy and safety of a novel approach to treat severe (grades III-IV) SR-aGVHD with the combination of basiliximab and etanercept. Sixty-five patients with severe SR-aGVHD from six centers were included. The median number of basiliximab infusions was 4 (range 2-11) and of etanercept was 9 (range 2-12). At day 28 after starting the combination treatment, overall response (complete and partial response: CR+PR) to second-line treatment was 90.8% with 75.4% being CR. The incidences of CR per organ were 100%, 73.8%, and 79.7% for skin, liver, and gut involvement, respectively. Patients >30-y old (p = 0.043, RR = 3.169), development of grades III-IV liver aGVHD (p = 0.007, RR = 5.034) and cytomegalovirus (CMV) reactivation (p = 0.035, RR = 4.02) were independent predictors for incomplete response. Combined treatment with basiliximab and etanercept resulted in improved CR to visceral aGVHD and significantly superior 2-y overall survival (54.7% vs. 14.8%, p <0.001) compared with classical salvage treatments. Our data suggest that the combination of basiliximab and etanercept may constitute a promising new treatment option for SR-aGVHD.
Keywords: Basiliximab; etanercept; hematopoietic stem cell transplantation; steroid-refractory acute GVHD.
Figures
Similar articles
-
Meta-Analysis of Interleukin-2 Receptor Antagonists as the Treatment for Steroid-Refractory Acute Graft-Versus-Host Disease.Front Immunol. 2021 Sep 21;12:749266. doi: 10.3389/fimmu.2021.749266. eCollection 2021. Front Immunol. 2021. PMID: 34621279 Free PMC article.
-
[Efficacy of basiliximab in the treatment of 87 cases of steroid-refractory or steroid-dependent acute graft-versus-host disease].Zhonghua Xue Ye Xue Za Zhi. 2022 Feb 14;43(2):120-127. doi: 10.3760/cma.j.issn.0253-2727.2022.02.006. Zhonghua Xue Ye Xue Za Zhi. 2022. PMID: 35381672 Free PMC article. Chinese.
-
Vedolizumab plus basiliximab as second-line therapy for steroid-refractory lower gastrointestinal acute graft-versus-host disease.Front Immunol. 2024 Jul 3;15:1408211. doi: 10.3389/fimmu.2024.1408211. eCollection 2024. Front Immunol. 2024. PMID: 39021571 Free PMC article.
-
Basiliximab as Treatment for Steroid-Refractory Acute Graft-versus-Host Disease in Pediatric Patients after Haploidentical Hematopoietic Stem Cell Transplantation.Biol Blood Marrow Transplant. 2020 Feb;26(2):351-357. doi: 10.1016/j.bbmt.2019.10.031. Epub 2019 Nov 5. Biol Blood Marrow Transplant. 2020. PMID: 31704470
-
Efficacy of Mesenchymal Stem Cell Therapy for Steroid-Refractory Acute Graft-Versus-Host Disease following Allogeneic Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis.PLoS One. 2015 Aug 31;10(8):e0136991. doi: 10.1371/journal.pone.0136991. eCollection 2015. PLoS One. 2015. PMID: 26323092 Free PMC article.
Cited by
-
Meta-Analysis of Interleukin-2 Receptor Antagonists as the Treatment for Steroid-Refractory Acute Graft-Versus-Host Disease.Front Immunol. 2021 Sep 21;12:749266. doi: 10.3389/fimmu.2021.749266. eCollection 2021. Front Immunol. 2021. PMID: 34621279 Free PMC article.
-
[Short-term substitution of calcineurin inhibitors (CNI) with recombinant humanized anti-CD25 monoclonal antibody (Basiliximab) as aGVHD prophylaxis in CNI intolerant patients after allogeneic hematopoietic stem cell transplantation].Zhonghua Xue Ye Xue Za Zhi. 2024 Feb 14;45(2):115-120. doi: 10.3760/cma.j.cn121090-20230519-00201. Zhonghua Xue Ye Xue Za Zhi. 2024. PMID: 38604786 Free PMC article. Chinese.
-
Mesenchymal stromal cells plus basiliximab improve the response of steroid-refractory acute graft-versus-host disease as a second-line therapy: a multicentre, randomized, controlled trial.BMC Med. 2024 Feb 27;22(1):85. doi: 10.1186/s12916-024-03275-5. BMC Med. 2024. PMID: 38413930 Free PMC article. Clinical Trial.
-
Established and Emerging Treatments of Skin GvHD.Front Immunol. 2022 Feb 2;13:838494. doi: 10.3389/fimmu.2022.838494. eCollection 2022. Front Immunol. 2022. PMID: 35185931 Free PMC article. Review.
-
[Efficacy of basiliximab in the treatment of 87 cases of steroid-refractory or steroid-dependent acute graft-versus-host disease].Zhonghua Xue Ye Xue Za Zhi. 2022 Feb 14;43(2):120-127. doi: 10.3760/cma.j.issn.0253-2727.2022.02.006. Zhonghua Xue Ye Xue Za Zhi. 2022. PMID: 35381672 Free PMC article. Chinese.
References
-
- Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet 2009; 373(9674):1550-61; PMID:19282026; http://dx.doi.org/10.1016/S0140-6736(09)60237-3 - DOI - PMC - PubMed
-
- Martin PJ, Schoch G, Fisher L, Byers V, Anasetti C, Appelbaum FR et al.. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood 1990; 76(8):1464-72; PMID:2207321 - PubMed
-
- Roy J, McGlave PB, Filipovich AH, Miller WJ, Blazar BR, Ramsay NK et al.. Acute graft-versus-host disease following unrelated donor marrow transplantation: failure of conventional therapy. Bone Marrow Transpl 1992; 10(1):77-82; PMID:1515883 - PubMed
-
- Deeg HJ. How I treat refractory acute GVHD. Blood 2007; 109(10):4119-26; PMID:17234737; http://dx.doi.org/10.1182/blood-2006-12-041889 - DOI - PMC - PubMed
-
- Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C et al.. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transpl 2012; 18(8):1150-63; PMID:22510384; http://dx.doi.org/10.1016/j.bbmt.2012.04.005 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials