Zoledronate can induce colorectal cancer microenvironment expressing BTN3A1 to stimulate effector γδ T cells with antitumor activity
- PMID: 28405500
- PMCID: PMC5384426
- DOI: 10.1080/2162402X.2016.1278099
Zoledronate can induce colorectal cancer microenvironment expressing BTN3A1 to stimulate effector γδ T cells with antitumor activity
Abstract
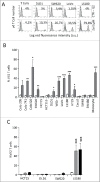
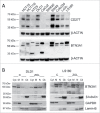
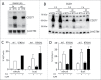
Amino-bis-phosphonates (N-BPs) such as zoledronate (Zol) have been used in anticancer clinical trials due to their ability to upregulate pyrophosphate accumulation promoting antitumor Vγ9Vδ2 T cells. The butyrophilin 3A (BTN3A, CD277) family, mainly the BTN3A1 isoform, has emerged as an important structure contributing to Vγ9Vδ2 T cells stimulation. It has been demonstrated that the B30.2 domain of BTN3A1 can bind phosphoantigens (PAg) and drive the activation of Vγ9Vδ2 T cells through conformational changes of the extracellular domains. Moreover, BTN3A1 binding to the cytoskeleton, and its consequent membrane stabilization, is crucial to stimulate the PAg-induced tumor cell reactivity by human Vγ9Vδ2 T cells. Aim of this study was to investigate the relevance of BTN3A1 in N-BPs-induced antitumor response in colorectal cancer (CRC) and the cell types involved in the tumor microenvironment. In this paper, we show that (i) CRC, exposed to Zol, stimulates the expansion of Vδ2 T lymphocytes with effector memory phenotype and antitumor cytotoxic activity, besides sensitizing cancer cells to γδ T cell-mediated cytotoxicity; (ii) this effect is partially related to BTN3A1 expression and in particular with its cellular re-distribution in the membrane and cytoskeleton-associated fraction; (iii) BTN3A1 is detected in CRC at the tumor site, both on epithelial cells and on tumor-associated fibroblasts (TAF), close to areas infiltrated by Vδ2 T lymphocytes; (iv) Zol is effective in stimulating antitumor effector Vδ2 T cells from ex-vivo CRC cell suspensions; and (v) both CRC cells and TAF can be primed by Zol to trigger Vδ2 T cells.
Keywords: Amino-bis-phosphonates; butyrophilin; colorectal cancer; immunostimulation; phosphate antigens.
Figures





References
-
- Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity 2009; 31(2):184-96; PMID:19699170; http://dx.doi.org/ 10.1016/j.immuni.2009.08.006 - DOI - PubMed
-
- Bonneville M, O'Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol 2010; 10(7):467-78; PMID:20539306; http://dx.doi.org/ 10.1038/nri2781 - DOI - PubMed
-
- Poggi A, Zocchi MR. γδ T lymphocytes as a first line of immune defense: Old and new ways of antigen recognition and implications for cancer immunotherapy. Front Immunol 2014; 5:575; PMID:25426121; http://dx.doi.org/ 10.3389/fimmu.2014.00575 eCollection 2014; http://www.jem.org/cgi/doi/ 10.1084/jem.20021500 - DOI - PMC - PubMed
-
- Gober HJ, Kistowska M, Angman L, Jenö P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med 2003; 197(2):163-8; PMID:12538656; http://www.jem.org/cgi/doi/ 10.1084/jem.20021500 - DOI - PMC - PubMed
-
- Wang H, Sarikonda G, Puan KJ, Tanaka Y, Feng J, Giner JL, Cao R, Mönkkönen J, Oldfield E, Morita CT. Indirect stimulation of human Vγ2Vδ2 T cells through alterations in isoprenoid metabolism. J Immunol 2011; 187(10):5099-113; PMID:22013129; http://dx.doi.org/ 10.4049/jimmunol.1002697 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
