Advanced glycation end products as a biomarker for incisional hernia
- PMID: 28405811
- PMCID: PMC5517588
- DOI: 10.1007/s10029-017-1610-2
Advanced glycation end products as a biomarker for incisional hernia
Abstract
Background: Incisional hernia is one of the most frequent complications after abdominal surgery, with incidences up to 30%. A reliable biomarker for the prediction of this complication is lacking. Advanced glycosylation end products (AGEs), also known as non-enzymatic collagen crosslinks, are correlated with aging, smoking, hyperglycemia, hyperlipidemia and oxidative stress. In this study the accumulation of AGEs and the relation between AGEs and incisional hernia were investigated.
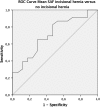
Materials and methods: In an exploratory case-control study, 23 patients with incisional hernia after midline incision were compared with 17 patients without clinical or radiological signs of incisional hernia after midline incision, AGEs were measured using a Skin Auto Fluorescence (SAF)-reader.
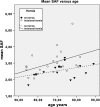
Results: Twenty-three patients with a clinically significant incisional hernia and 17 control patients were included. The study groups had significant differences in mean BMI. There was a significant difference between mean AGEs in patients with and without incisional hernia after midline incision (3.00 ± 0.15 vs. 2.56 ± 0.11, T test p = 0.03).
Conclusion: AGE accumulation measured in the skin indirectly with autofluorescence might be associated with incisional hernia. Prospective larger trials should confirm this finding.
Keywords: Advanced glycation endproducts; Biomarker; Collagen cross links; Incisional hernia.
Conflict of interest statement
Conflict of interest
J.J. Harlaar, declares no conflict of interest H.H. Eker declares no conflict of interest K.A. Vakalopoulos declares no conflict of interest M Castro Cabezas declares no conflict of interest A.C. van der Ham declares no conflict of interest W.W. Vrijland declares no conflict of interest J. Jeekel declares no conflict of interest J.F. Lange declares no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human and animal rights
Compliance with ethical standards was adhered to Institutional Review and Board approval.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures
References
-
- Deerenberg EB, Harlaar JJ, Steyerberg EW, Lont HE, van Doorn HC, Heisterkamp J, et al. Small bites versus large bites for closure of abdominal midline incisions (STITCH): a double-blind, multicentre, randomised controlled trial. Lancet. 2015;386(10000):1254–1260. doi: 10.1016/S0140-6736(15)60459-7. - DOI - PubMed
-
- Timmermans L, Eker HH, Steyerberg EW, Jairam A, de Jong D, Pierik EG, et al. Short-term results of a randomized controlled trial comparing primary suture with primary glued mesh augmentation to prevent incisional hernia. Ann Surg. 2015;261(2):276–281. doi: 10.1097/SLA.0000000000000798. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources