Estimating the prevalence of functional exonic splice regulatory information
- PMID: 28405812
- PMCID: PMC5602102
- DOI: 10.1007/s00439-017-1798-3
Estimating the prevalence of functional exonic splice regulatory information
Abstract
In addition to coding information, human exons contain sequences necessary for correct splicing. These elements are known to be under purifying selection and their disruption can cause disease. However, the density of functional exonic splicing information remains profoundly uncertain. Several groups have experimentally investigated how mutations at different exonic positions affect splicing. They have found splice information to be distributed widely in exons, with one estimate putting the proportion of splicing-relevant nucleotides at >90%. These results suggest that splicing could place a major pressure on exon evolution. However, analyses of sequence conservation have concluded that the need to preserve splice regulatory signals only slightly constrains exon evolution, with a resulting decrease in the average human rate of synonymous evolution of only 1-4%. Why do these two lines of research come to such different conclusions? Among other reasons, we suggest that the methods are measuring different things: one assays the density of sites that affect splicing, the other the density of sites whose effects on splicing are visible to selection. In addition, the experimental methods typically consider short exons, thereby enriching for nucleotides close to the splice junction, such sites being enriched for splice-control elements. By contrast, in part owing to correction for nucleotide composition biases and to the assumption that constraint only operates on exon ends, the conservation-based methods can be overly conservative.
Keywords: Exon Inclusion; Exonic Splice; Motif Density; Splice Assay; Synonymous Site.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures
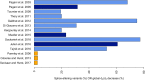

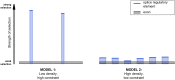
Similar articles
-
Compensatory relationship between splice sites and exonic splicing signals depending on the length of vertebrate introns.BMC Genomics. 2006 Dec 8;7:311. doi: 10.1186/1471-2164-7-311. BMC Genomics. 2006. PMID: 17156453 Free PMC article.
-
Exonic splice regulation imposes strong selection at synonymous sites.Genome Res. 2018 Oct;28(10):1442-1454. doi: 10.1101/gr.233999.117. Epub 2018 Aug 24. Genome Res. 2018. PMID: 30143596 Free PMC article.
-
Determinants of the Usage of Splice-Associated cis-Motifs Predict the Distribution of Human Pathogenic SNPs.Mol Biol Evol. 2016 Feb;33(2):518-29. doi: 10.1093/molbev/msv251. Epub 2015 Nov 5. Mol Biol Evol. 2016. PMID: 26545919 Free PMC article.
-
Searching for splicing motifs.Adv Exp Med Biol. 2007;623:85-106. doi: 10.1007/978-0-387-77374-2_6. Adv Exp Med Biol. 2007. PMID: 18380342 Review.
-
Exonization of transposed elements: A challenge and opportunity for evolution.Biochimie. 2011 Nov;93(11):1928-34. doi: 10.1016/j.biochi.2011.07.014. Epub 2011 Jul 26. Biochimie. 2011. PMID: 21787833 Review.
Cited by
-
RES complex is associated with intron definition and required for zebrafish early embryogenesis.PLoS Genet. 2018 Jul 3;14(7):e1007473. doi: 10.1371/journal.pgen.1007473. eCollection 2018 Jul. PLoS Genet. 2018. PMID: 29969449 Free PMC article.
-
Distinct functional consequences of ECEL1/DINE missense mutations in the pathogenesis of congenital contracture disorders.Acta Neuropathol Commun. 2017 Nov 13;5(1):83. doi: 10.1186/s40478-017-0486-9. Acta Neuropathol Commun. 2017. PMID: 29132416 Free PMC article.
-
Exonic mutations and exon skipping: Lessons learned from DFNA5.Hum Mutat. 2018 Mar;39(3):433-440. doi: 10.1002/humu.23384. Epub 2018 Jan 11. Hum Mutat. 2018. PMID: 29266521 Free PMC article.
-
Evidence in disease and non-disease contexts that nonsense mutations cause altered splicing via motif disruption.Nucleic Acids Res. 2021 Sep 27;49(17):9665-9685. doi: 10.1093/nar/gkab750. Nucleic Acids Res. 2021. PMID: 34469537 Free PMC article.
-
Splicing in the Diagnosis of Rare Disease: Advances and Challenges.Front Genet. 2021 Jul 1;12:689892. doi: 10.3389/fgene.2021.689892. eCollection 2021. Front Genet. 2021. PMID: 34276790 Free PMC article. Review.
References
-
- Amit M, et al. Differential GC content between exons and introns establishes distinct strategies of splice-site recognition. Cell Rep. 2012;1:543–556. - PubMed
-
- Amundson R, Lauder GV. Function without purpose: the uses of causal role function in evolutionary biology. Biol Philos. 1994;9:443–469.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

