Everolimus as first line therapy for pancreatic neuroendocrine tumours: current knowledge and future perspectives
- PMID: 28405826
- PMCID: PMC11819053
- DOI: 10.1007/s00432-017-2407-5
Everolimus as first line therapy for pancreatic neuroendocrine tumours: current knowledge and future perspectives
Abstract
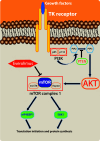
Purpose: Everolimus has been shown to be effective for advanced pancreatic neuroendocrine tumours (pNETs), but its positioning in the therapeutic algorithm for pNETs is matter of debate.
Methods: With the aim to shed light on this point, we performed an up-to-date critical review taking into account the results of both retrospective and prospective published studies, and the recommendations of international guidelines. In addition, we performed an extensive search on the Clinical Trial Registries databases worldwide, to gather information on the ongoing clinical trials related to this specific topic.
Results: We identified eight retrospective published studies, two prospective published studies, and five registered clinical trials. Moreover, we analyzed the content of four widespread international guidelines.
Conclusions: Our critical review confirms the lack of high-quality data to recommend everolimus as the first line therapy for pNETs. The ongoing clinical trials reported in this review will hopefully help clinicians, in the near future, to better evaluate the role of everolimus as the first line therapy for pNETs. However, at the moment, there is already enough evidence to recommend everolimus as the first line therapy for patients with symptomatic malignant unresectable insulin-secreting pNETs, to control the endocrine syndrome regardless of tumour growth.
Keywords: Everolimus; Neuroendocrine tumours; Therapy; mTOR inhibitors.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures
References
-
- Altomare DA, Testa JR (2005) Perturbations of the AKT signaling pathway in human cancer. Oncogene 24:7455–7464. doi:10.1038/sj.onc.1209085 - PubMed
-
- Anthony LB et al (2015) Impact of previous somatostatin analogue use on the activity of everolimus in patients with advanced neuroendocrine tumors: analysis from the phase III RADIANT-2 Trial. Neuroendocrinology 102:18–25. doi:10.1159/000381715 - PubMed
-
- Bajetta E et al (2014) Everolimus in combination with octreotide long-acting repeatable in a first-line setting for patients with neuroendocrine tumors: an ITMO group study. Cancer 120:2457–2463. doi:10.1002/cncr.28726 - PubMed
-
- Benslama N et al (2016) Prediction of response to everolimus in neuroendocrine tumors: evaluation of clinical, biological and histological factors. Invest New Drugs doi:10.1007/s10637-016-0363-6 - PubMed
-
- Bernard V et al (2013) Efficacy of everolimus in patients with metastatic insulinoma and refractory hypoglycemia. Eur J Endocrinol 168:665–674 doi:10.1530/EJE-12-1101 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous