Exploiting Uniformly 13C-Labeled Carbohydrates for Probing Carbohydrate-Protein Interactions by NMR Spectroscopy
- PMID: 28406013
- PMCID: PMC5725960
- DOI: 10.1021/jacs.7b01929
Exploiting Uniformly 13C-Labeled Carbohydrates for Probing Carbohydrate-Protein Interactions by NMR Spectroscopy
Abstract
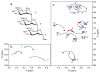
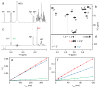
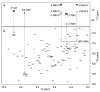
NMR of a uniformly 13C-labeled carbohydrate was used to elucidate the atomic details of a sugar-protein complex. The structure of the 13C-labeled Manα(1-2)Manα(1-2)ManαOMe trisaccharide ligand, when bound to cyanovirin-N (CV-N), was characterized and revealed that in the complex the glycosidic linkage torsion angles between the two reducing-end mannoses are different from the free trisaccharide. Distances within the carbohydrate were employed for conformational analysis, and NOE-based distance mapping between sugar and protein revealed that Manα(1-2)Manα(1-2)ManαOMe is bound more intimately with its two reducing-end mannoses into the domain A binding site of CV-N than with the nonreducing end unit. Taking advantage of the 13C spectral dispersion of 13C-labeled carbohydrates in isotope-filtered experiments is a versatile means for a simultaneous mapping of the binding interactions on both, the carbohydrate and the protein.
Figures

Similar articles
-
Atomic mapping of the sugar interactions in one-site and two-site mutants of cyanovirin-N by NMR spectroscopy.Biochemistry. 2008 Mar 25;47(12):3625-35. doi: 10.1021/bi702200m. Epub 2008 Mar 1. Biochemistry. 2008. PMID: 18311923
-
Atomic mapping of the interactions between the antiviral agent cyanovirin-N and oligomannosides by saturation-transfer difference NMR.Biochemistry. 2004 Nov 9;43(44):13926-31. doi: 10.1021/bi048676k. Biochemistry. 2004. PMID: 15518540
-
Direct Observation of Carbohydrate Hydroxyl Protons in Hydrogen Bonds with a Protein.J Am Chem Soc. 2018 Jan 10;140(1):339-345. doi: 10.1021/jacs.7b10595. Epub 2017 Dec 22. J Am Chem Soc. 2018. PMID: 29227646
-
A perspective on the primary and three-dimensional structures of carbohydrates.Carbohydr Res. 2013 Aug 30;378:123-32. doi: 10.1016/j.carres.2013.02.005. Epub 2013 Feb 24. Carbohydr Res. 2013. PMID: 23522728 Review.
-
Detection of intermolecular NOE interactions in large protein complexes.Prog Nucl Magn Reson Spectrosc. 2016 Nov;97:40-56. doi: 10.1016/j.pnmrs.2016.08.002. Epub 2016 Aug 18. Prog Nucl Magn Reson Spectrosc. 2016. PMID: 27888839 Review.
Cited by
-
Novel NMR Avenues to Explore the Conformation and Interactions of Glycans.ACS Omega. 2019 Aug 19;4(9):13618-13630. doi: 10.1021/acsomega.9b01901. eCollection 2019 Aug 27. ACS Omega. 2019. PMID: 31497679 Free PMC article. Review.
-
Solid-State NMR of highly 13C-enriched cholesterol in lipid bilayers.Methods. 2018 Apr 1;138-139:47-53. doi: 10.1016/j.ymeth.2018.01.008. Epub 2018 Feb 21. Methods. 2018. PMID: 29366688 Free PMC article.
-
Selective 13 C-Labels on Repeating Glycan Oligomers to Reveal Protein Binding Epitopes through NMR: Polylactosamine Binding to Galectins.Angew Chem Int Ed Engl. 2021 Aug 16;60(34):18777-18782. doi: 10.1002/anie.202106056. Epub 2021 Jul 13. Angew Chem Int Ed Engl. 2021. PMID: 34128568 Free PMC article.
-
Harnessing 13C-labeled myo-inositol to interrogate inositol phosphate messengers by NMR.Chem Sci. 2019 Apr 23;10(20):5267-5274. doi: 10.1039/c9sc00151d. eCollection 2019 May 28. Chem Sci. 2019. PMID: 31191882 Free PMC article.
-
Automated Synthesis of Algal Fucoidan Oligosaccharides.J Am Chem Soc. 2024 Jul 10;146(27):18320-18330. doi: 10.1021/jacs.4c02348. Epub 2024 Jun 25. J Am Chem Soc. 2024. PMID: 38916244 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

