Exploiting Uniformly 13C-Labeled Carbohydrates for Probing Carbohydrate-Protein Interactions by NMR Spectroscopy
- PMID: 28406013
- PMCID: PMC5725960
- DOI: 10.1021/jacs.7b01929
Exploiting Uniformly 13C-Labeled Carbohydrates for Probing Carbohydrate-Protein Interactions by NMR Spectroscopy
Abstract
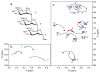
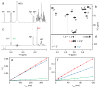
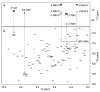
NMR of a uniformly 13C-labeled carbohydrate was used to elucidate the atomic details of a sugar-protein complex. The structure of the 13C-labeled Manα(1-2)Manα(1-2)ManαOMe trisaccharide ligand, when bound to cyanovirin-N (CV-N), was characterized and revealed that in the complex the glycosidic linkage torsion angles between the two reducing-end mannoses are different from the free trisaccharide. Distances within the carbohydrate were employed for conformational analysis, and NOE-based distance mapping between sugar and protein revealed that Manα(1-2)Manα(1-2)ManαOMe is bound more intimately with its two reducing-end mannoses into the domain A binding site of CV-N than with the nonreducing end unit. Taking advantage of the 13C spectral dispersion of 13C-labeled carbohydrates in isotope-filtered experiments is a versatile means for a simultaneous mapping of the binding interactions on both, the carbohydrate and the protein.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

