Optical Coherence Tomography Angiography to Estimate Retinal Blood Flow in Eyes with Retinitis Pigmentosa
- PMID: 28406171
- PMCID: PMC5390317
- DOI: 10.1038/srep46396
Optical Coherence Tomography Angiography to Estimate Retinal Blood Flow in Eyes with Retinitis Pigmentosa
Abstract
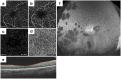
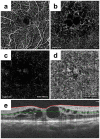
Ophthalmologists sometimes face difficulties in identifying the origin of visual acuity (VA) loss in a retinitis pigmentosa (RP) patient, particularly before cataract surgery: cataract or the retinal disease state. Therefore, it is important to identify the significant factors correlating with VA. Nowadays, retinal blood flow in superficial and deep layers can be estimated non-invasively using optical coherence tomography angiography (OCTA). We estimated blood flow per retinal layer by using OCTA; investigated the correlation between VA and other parameters including blood flow and retinal thickness; and identified the most associated factor with VA in patients with RP. OCTA images in 68 of consecutive 110 Japanese RP patients were analysable (analysable RP group). Thirty-two age- and axial length-matched healthy eyes (control group) were studied. In the analysable RP group, the parafoveal flow density in superficial and deep layers was 47.0 ± 4.9% and 52.4 ± 5.5%, respectively, which was significantly lower than that in controls. Using multivariate analysis, we found that the parafoveal flow density in the deep layer and superficial foveal avascular area were the factors associated with VA. Non-invasive estimation of retinal blood flow per retinal layer using OCTA is useful for predicting VA in RP patients.
Conflict of interest statement
There are potential competing financial interests. Nagahisa Yoshimura has received financial support from Topcon Corporation, Nidek, and Canon (unrelated to this study), and has provided consultancy services to Nidek. There are no patents, products in development, or marketed products to declare. The other authors declare no competing interests.
Figures



References
-
- Hartong D. T., Berson E. L. & Dryja T. P. Retinitis pigmentosa. Lancet. 368, 1795–1809 (2006). - PubMed
-
- Yoshida N. et al.. Factors affecting visual acuity after cataract surgery in patients with retinitis pigmentosa. Ophthalmology. 122, 903–908 (2015). - PubMed
-
- Krill A. E., Archer D. & Newell F. W. Fluorescein angiography in retinitis pigmentosa. Am. J. Ophthalmol. 69, 826–835 (1970). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

