Local inhibition of microtubule dynamics by dynein is required for neuronal cargo distribution
- PMID: 28406181
- PMCID: PMC5399302
- DOI: 10.1038/ncomms15063
Local inhibition of microtubule dynamics by dynein is required for neuronal cargo distribution
Abstract
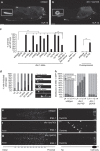
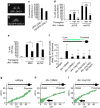
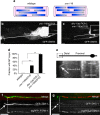
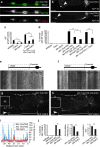
Abnormal axonal transport is associated with neuronal disease. We identified a role for DHC-1, the C. elegans dynein heavy chain, in maintaining neuronal cargo distribution. Surprisingly, this does not involve dynein's role as a retrograde motor in cargo transport, hinging instead on its ability to inhibit microtubule (MT) dynamics. Neuronal MTs are highly static, yet the mechanisms and functional significance of this property are not well understood. In disease-mimicking dhc-1 alleles, excessive MT growth and collapse occur at the dendrite tip, resulting in the formation of aberrant MT loops. These unstable MTs act as cargo traps, leading to ectopic accumulations of cargo and reduced availability of cargo at normal locations. Our data suggest that an anchored dynein pool interacts with plus-end-out MTs to stabilize MTs and allow efficient retrograde transport. These results identify functional significance for neuronal MT stability and suggest a mechanism for cellular dysfunction in dynein-linked disease.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Conde C. & Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 10, 319–332 (2009). - PubMed
-
- Janke C. & Kneussel M. Tubulin post-translational modifications: encoding functions on the neuronal microtubule cytoskeleton. Trends Neurosci. 33, 362–372 (2010). - PubMed
-
- Kapitein L. C. & Hoogenraad C. C. Building the neuronal microtubule cytoskeleton. Neuron 87, 492–506 (2015). - PubMed
-
- Coles C. H. & Bradke F. Coordinating neuronal actin-microtubule dynamics. Curr. Biol. 25, R677–R691 (2015). - PubMed
-
- Millecamps S. & Julien J. P. Axonal transport deficits and neurodegenerative diseases. Nat. Rev. Neurosci. 14, 161–176 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

