Topological defects in epithelia govern cell death and extrusion
- PMID: 28406198
- PMCID: PMC5439518
- DOI: 10.1038/nature21718
Topological defects in epithelia govern cell death and extrusion
Abstract
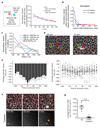
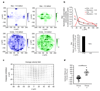
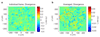
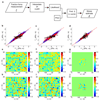
Epithelial tissues (epithelia) remove excess cells through extrusion, preventing the accumulation of unnecessary or pathological cells. The extrusion process can be triggered by apoptotic signalling, oncogenic transformation and overcrowding of cells. Despite the important linkage of cell extrusion to developmental, homeostatic and pathological processes such as cancer metastasis, its underlying mechanism and connections to the intrinsic mechanics of the epithelium are largely unexplored. We approach this problem by modelling the epithelium as an active nematic liquid crystal (that has a long range directional order), and comparing numerical simulations to strain rate and stress measurements within monolayers of MDCK (Madin Darby canine kidney) cells. Here we show that apoptotic cell extrusion is provoked by singularities in cell alignments in the form of comet-shaped topological defects. We find a universal correlation between extrusion sites and positions of nematic defects in the cell orientation field in different epithelium types. The results confirm the active nematic nature of epithelia, and demonstrate that defect-induced isotropic stresses are the primary precursors of mechanotransductive responses in cells, including YAP (Yes-associated protein) transcription factor activity, caspase-3-mediated cell death, and extrusions. Importantly, the defect-driven extrusion mechanism depends on intercellular junctions, because the weakening of cell-cell interactions in an α-catenin knockdown monolayer reduces the defect size and increases both the number of defects and extrusion rates, as is also predicted by our model. We further demonstrate the ability to control extrusion hotspots by geometrically inducing defects through microcontact printing of patterned monolayers. On the basis of these results, we propose a mechanism for apoptotic cell extrusion: spontaneously formed topological defects in epithelia govern cell fate. This will be important in predicting extrusion hotspots and dynamics in vivo, with potential applications to tissue regeneration and the suppression of metastasis. Moreover, we anticipate that the analogy between the epithelium and active nematic liquid crystals will trigger further investigations of the link between cellular processes and the material properties of epithelia.
Conflict of interest statement
Authors do not have conflict of interests to declare.
Figures





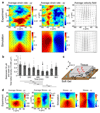
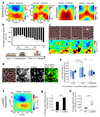

Comment in
-
The 100-year-old challenge to Darwin that is still making waves in research.Nature. 2017 Apr 12;544(7649):138-139. doi: 10.1038/544138a. Nature. 2017. PMID: 28406221 No abstract available.
References
-
- Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin-and myosin-dependent mechanism. Curr Biol. 2001;11:1847–1857. - PubMed
-
- Hogan C, et al. Characterization of the interface between normal and transformed epithelial cells. Nat Cell Biol. 2009;11:460–467. - PubMed
-
- Marinari E, et al. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature. 2012;484:542–545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

