Pharmacotherapy for hyperuricemia in hypertensive patients
- PMID: 28406263
- PMCID: PMC6478066
- DOI: 10.1002/14651858.CD008652.pub3
Pharmacotherapy for hyperuricemia in hypertensive patients
Update in
-
Pharmacotherapy for hyperuricaemia in hypertensive patients.Cochrane Database Syst Rev. 2020 Sep 2;9(9):CD008652. doi: 10.1002/14651858.CD008652.pub4. Cochrane Database Syst Rev. 2020. PMID: 32877573 Free PMC article.
Abstract
Background: High blood pressure represents a major public health problem. Worldwide, approximately one-fourth of the adult population has hypertension. Epidemiological and experimental studies suggest a link between hyperuricemia and hypertension. Hyperuricemia affects 25% to 40 % of individuals with untreated hypertension; a much lower prevalence has been reported in normotensives or in the general population. However, whether lowering serum uric acid (UA) might lower blood pressure (BP) is an unanswered question.
Objectives: To determine whether UA-lowering agents reduce BP in patients with primary hypertension or prehypertension compared with placebo.
Search methods: The Cochrane Hypertension Information Specialist searched the following databases for randomized controlled trials up to February 2016: the Cochrane Hypertension Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (2016, Issue 2), MEDLINE (from 1946), Embase (from 1974), the World Health Organization International Clinical Trials Registry Platform, and ClinicalTrials.gov. We also searched LILACS up to March 2016 and contacted authors of relevant papers regarding further published and unpublished work.
Selection criteria: To be included in this review, the studies had to meet the following criteria: 1) randomized or quasi-randomized, with a group assigned to receive a UA-lowering agent and another group assigned to receive placebo; 2) double-blind, single-blind or open-label; 3) parallel or cross-over trial; 4) cross-over trials had to have a washout period of at least two weeks; 5) minimum treatment duration of four weeks; 6) participants had to have a diagnosis of essential hypertension or prehypertension, and hyperuricemia (serum UA greater than 6 mg/dL in women, 7 mg/dL in men and 5.5 mg/dL in children/adolescents); 7) outcome measures assessed included change in clinic systolic, diastolic or 24-hour ambulatory BP.
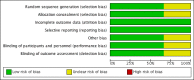
Data collection and analysis: The two review authors independently collected the data using a data extraction form, and resolved any disagreements via discussion. We assessed risk of bias using the Cochrane Collaboration' Risk of bias' tool.
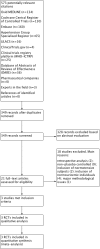
Main results: In this review update, we examined the abstracts of 349 identified papers and selected 21 for evaluation. We also identified three ongoing studies, the results of which are not yet available. Three other randomized controlled trials (RCTs) (two new), enrolling individuals with hypertension or prehypertension, and hyperuricemia, met the inclusion criteria for the review and were included in the meta-analysis. Low quality of evidence from three RCTs indicate no reduction in systolic (MD -6.2 mmHg, 95% CI -12.8 to 0.5) or diastolic (-3.9 mmHg, 95% CI -9.2 to 1.4) 24-hour ambulatory BP with UA-lowering drugs compared with placebo. Low quality of evidence from two RCTs reveal a reduction of systolic clinic BP (-8.43 mmHg, 95% CI -15.24 to -1.62) but not diastolic clinic BP (-6.45 mmHg, 95% CI -13.60 to 0.70). High quality of evidence from three RCTs indicates that serum UA levels were reduced by 3.1 mg/dL (95% CI 2.4 to 3.8) in the participants that received UA-lowering drugs. Very low quality of evidence from three RCTs suggests that withdrawals due to adverse effects were not increased with UA-lowering therapy (RR 1.86, 95% CI 0.43 to 8.10).
Authors' conclusions: In this updated systematic review, the RCT data available at present are insufficient to know whether UA-lowering therapy also lowers BP. More studies are needed.
Conflict of interest statement
Nothing to declare.
Figures





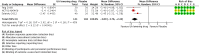







Update of
-
Pharmacotherapy for hyperuricemia in hypertensive patients.Cochrane Database Syst Rev. 2013 Jan 31;(1):CD008652. doi: 10.1002/14651858.CD008652.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2017 Apr 13;4:CD008652. doi: 10.1002/14651858.CD008652.pub3. PMID: 23440832 Updated.
References
References to studies included in this review
-
- NCT01496469. Effect of febuxostat on blood pressure [A phase 2, double‐blind, placebo‐controlled study to assess the effect of febuxostat 80 mg once daily compared to placebo on ambulatory blood pressure in subjects with hyperuricemia and hypertension]. clinicaltrials.gov/ct2/show/NCT01496469 (first received 18 December 2011).
-
- Soletsky B, Feig DI. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension 2012;60(5):1148‐56. - PubMed
References to studies excluded from this review
-
- Assadi F. Allopurinol enhances the blood pressure lowering effect of enalapril in children with hyperuricemic essential hypertension. Journal of Nephrology 2014;27(1):51‐6. - PubMed
-
- Feig DI, Nakagawa T, Karumanchi SA, Oliver WJ, Kang DH, Finch J, et al. Hypothesis: uric acid, nephron number, and the pathogenesis of essential hypertension. Kidney International 2004;66(1):281‐7. - PubMed
-
- Higgins P, Walters MR, Murray HM, McArthur K, McConnachie A, Lees KR, et al. Allopurinol reduces brachial and central blood pressure, and carotid intima‐media thickness progression after ischaemic stroke and transient ischaemic attack: a randomised controlled trial. Heart 2014;100(14):1085‐92. - PubMed
References to ongoing studies
-
- Influence of XOI, febuxostat, on vascular function in patients with hyperuricemia and cardiovascular risk factors.. Ongoing study October 2015..
-
- The effect of allopurinol on serum uric acid level and arterial blood pressure in hemodialysis patients.. Ongoing study December 2014..
-
- The effect of the combination of antihypertensive and urate‐lowering therapy on vascular endothelial function in patients with hypertensive and asymptomatic hyperuricemia.. Ongoing study December 2013.
Additional references
-
- Alper AB Jr, Chen W, Yau L, Srinivasan SR, Berenson GS, Hamm LL. Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension 2005;45(1):34‐8. - PubMed
-
- Cannon PJ, Stason WB, Demartini FE, Sommers SC, Laragh JH. Hyperuricemia in primary and renal hypertension. New England Journal of Medicine 1966;275(9):457–64. - PubMed
-
- Carretero OA, Oparil S. Essential hypertension: part I: definition and etiology. Circulation 2000;101(3):329‐35. - PubMed
-
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure: the JNC 7 report. Journal of the American Medical Association 2003;289(19):2560‐72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

