Brief Report: Robo1 Regulates the Migration of Human Subventricular Zone Neural Progenitor Cells During Development
- PMID: 28406573
- PMCID: PMC5484745
- DOI: 10.1002/stem.2628
Brief Report: Robo1 Regulates the Migration of Human Subventricular Zone Neural Progenitor Cells During Development
Abstract
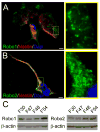
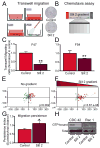
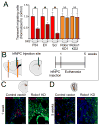
Human neural progenitor cell (NPC) migration within the subventricular zone (SVZ) of the lateral ganglionic eminence is an active process throughout early brain development. The migration of human NPCs from the SVZ to the olfactory bulb during fetal stages resembles what occurs in adult rodents. As the human brain develops during infancy, this migratory stream is drastically reduced in cell number and becomes barely evident in adults. The mechanisms regulating human NPC migration are unknown. The Slit-Robo signaling pathway has been defined as a chemorepulsive cue involved in axon guidance and neuroblast migration in rodents. Slit and Robo proteins expressed in the rodent brain help guide neuroblast migration from the SVZ through the rostral migratory stream to the olfactory bulb. Here, we present the first study on the role that Slit and Robo proteins play in human-derived fetal neural progenitor cell migration (hfNPC). We describe that Robo1 and Robo2 isoforms are expressed in the human fetal SVZ. Furthermore, we demonstrate that Slit2 is able to induce a chemorepellent effect on the migration of hfNPCs derived from the human fetal SVZ. In addition, when Robo1 expression is inhibited, hfNPCs are unable to migrate to the olfactory bulb of mice when injected in the anterior SVZ. Our findings indicate that the migration of human NPCs from the SVZ is partially regulated by the Slit-Robo axis. This pathway could be regulated to direct the migration of NPCs in human endogenous neural cell therapy. Stem Cells 2017;35:1860-1865.
Keywords: Cell migration; Human subventricular zone; Neural stem cells; Slit2.
© 2017 AlphaMed Press.
Figures

References
-
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271(5251):978–81. - PubMed
-
- Curtis MA, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315(5816):1243–9. - PubMed
-
- Quinones-Hinojosa A, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494(3):415–34. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

