A Randomized Controlled Trial of Corticosteroids in Pediatric Septic Shock: A Pilot Feasibility Study
- PMID: 28406862
- PMCID: PMC5457353
- DOI: 10.1097/PCC.0000000000001121
A Randomized Controlled Trial of Corticosteroids in Pediatric Septic Shock: A Pilot Feasibility Study
Abstract
Objective: To determine the feasibility of conducting a randomized controlled trial of corticosteroids in pediatric septic shock.
Design: Randomized, double-blind, placebo controlled trial.
Setting: Seven tertiary level PICUs in Canada.
Patients: Children newborn to 17 years old inclusive with suspected septic shock.
Intervention: Administration of IV hydrocortisone versus placebo until hemodynamic stability is achieved or for a maximum of 7 days.
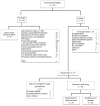
Measurements and main results: One hundred seventy-four patients were potentially eligible of whom 101 patients met eligibility criteria. Fifty-seven patients were randomized, and 49 patients (23 and 26 patients in the hydrocortisone and placebo groups, respectively) were included in the final analysis. The mean time from screening to randomization was 2.4 ± 2.1 hours and from screening to first dose of study drug was 3.8 ± 2.6 hours. Forty-two percent of potentially eligible patients (73/174) received corticosteroids prior to randomization: 38.5% (67/174) were already on corticosteroids for shock at the time of screening, and in 3.4% (6/174), the treating physician wished to administer corticosteroids. Six of 49 randomized patients (12.2%) received open-label steroids, three in each of the hydrocortisone and placebo groups. Time on vasopressors, days on mechanical ventilation, PICU and hospital length of stay, and the rate of adverse events were not statistically different between the two groups.
Conclusions: This study suggests that a large randomized controlled trial on early use of corticosteroids in pediatric septic shock is potentially feasible. However, the frequent use of empiric corticosteroids in otherwise eligible patients remains a significant challenge. Knowledge translation activities, targeted recruitment, and alternative study designs are possible strategies to mitigate this challenge.
Figures

Comment in
-
Randomized Clinical Trials of Corticosteroids in Septic Shock: Possibly Feasible, But Will They or Should They Change My Practice?Pediatr Crit Care Med. 2017 Jun;18(6):589-590. doi: 10.1097/PCC.0000000000001138. Pediatr Crit Care Med. 2017. PMID: 28574904 No abstract available.
-
Assessing Feasibility of Randomized Controlled Trials of Corticosteroids in Pediatric Septic Shock in Developed Countries: Only Half the Answer to the Problem.Pediatr Crit Care Med. 2017 Nov;18(11):1087. doi: 10.1097/PCC.0000000000001310. Pediatr Crit Care Med. 2017. PMID: 29099458 No abstract available.
-
The author replies.Pediatr Crit Care Med. 2017 Nov;18(11):1088. doi: 10.1097/PCC.0000000000001313. Pediatr Crit Care Med. 2017. PMID: 29099459 No abstract available.
References
-
- Menon K, McNally JD, Choong K, et al. A Cohort Study of Pediatric Shock: Frequency of Corticosteriod Use and Association with Clinical Outcomes. Shock. 2015;44:402–9. - PubMed
-
- Kissoon N, Carcillo JA, Espinosa V, et al. World Federation of Pediatric Intensive Care and Critical Care Societies: Global Sepsis Initiative. Pediatr Crit Care Med. 2011;12:494–503. - PubMed
-
- Schlapbach LJ, Straney L, Alexander J, et al. Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002–13: a multicentre retrospective cohort study. Lancet Infect Dis. 2015;15:46–54. - PubMed
-
- Valoor HT, Singhi S, Jayashree M. Low-dose hydrocortisone in pediatric septic shock: an exploratory study in a third world setting. Pediatr Crit Care Med. 2009;10:121–5. - PubMed
-
- Slusher T, Gbadero D, Howard C, et al. Randomized, placebo-controlled, double blinded trial of dexamethasone in African children with sepsis. Pediatr Infect Dis J. 1996;15:579–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

