Protection induced by virus-like particles containing Toxoplasma gondii microneme protein 8 against highly virulent RH strain of Toxoplasma gondii infection
- PMID: 28406951
- PMCID: PMC5391012
- DOI: 10.1371/journal.pone.0175644
Protection induced by virus-like particles containing Toxoplasma gondii microneme protein 8 against highly virulent RH strain of Toxoplasma gondii infection
Abstract
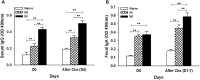
Toxoplasma gondii (T. gondii) microneme protein 8 (MIC8) represents a novel, functional distinct invasion factor. In this study, we generated virus-like particles (VLPs) targeting Toxoplasma gondii MIC8 for the first time, and investigated the protection against highly virulent RH strain of T. gondii in a mouse model. We found that VLP vaccination induced Toxoplasma gondii-specific IgG and IgG1 antibody responses in the sera. Upon challenge infection with RH strain of T. gondii tachyzoites, vaccinated mice showed a significant increase of both IgG antibodies in sera and IgA antibodies in feces compared to those before challenge, and a rapid expansion of both germinal center B cell (B220+, GL7+) and T cell (CD4+, CD8+) populations. Importantly, intranasally immunized mice showed higher neutralizing antibodies and displayed no proinflammatory cytokine IFN-γ in the spleen. Mice were completely protected from a lethal challenge infection with the highly virulent T. gondii (RH) showing no body weight loss (100% survival). Our study shows the effective protection against T. gondii infection provided by VLPs containing microneme protein 8 of T. gondii, thus indicating a potential T. gondii vaccine candidate.
Conflict of interest statement
Figures





Similar articles
-
Virus-like particle vaccines expressing Toxoplasma gondii rhoptry protein 18 and microneme protein 8 provide enhanced protection.Vaccine. 2018 Sep 11;36(38):5692-5700. doi: 10.1016/j.vaccine.2018.08.016. Epub 2018 Aug 11. Vaccine. 2018. PMID: 30107996
-
Virus-Like Nanoparticle Vaccine Confers Protection against Toxoplasma gondii.PLoS One. 2016 Aug 22;11(8):e0161231. doi: 10.1371/journal.pone.0161231. eCollection 2016. PLoS One. 2016. PMID: 27548677 Free PMC article.
-
Virus-like Particle Vaccine Containing Toxoplasma gondii Rhoptry Protein 13 Induces Protection against T. gondii ME49 Infection in Mice.Korean J Parasitol. 2019 Oct;57(5):543-547. doi: 10.3347/kjp.2019.57.5.543. Epub 2019 Oct 31. Korean J Parasitol. 2019. PMID: 31715698 Free PMC article.
-
Contribution of 65-kDa heat shock protein induced by gamma and delta T cells to protection against Toxoplasma gondii infection.Immunol Res. 1996;15(3):258-64. doi: 10.1007/BF02918253. Immunol Res. 1996. PMID: 8902580 Review.
-
Toxoplasma gondii and the Immunity-Related GTPase (IRG) resistance system in mice: a review.Mem Inst Oswaldo Cruz. 2009 Mar;104(2):234-40. doi: 10.1590/s0074-02762009000200016. Mem Inst Oswaldo Cruz. 2009. PMID: 19430648 Review.
Cited by
-
Protective Efficacy Induced by Virus-like Particles Expressing Dense Granule Protein 5 of Toxoplasma gondii.Vaccines (Basel). 2025 Jul 24;13(8):787. doi: 10.3390/vaccines13080787. Vaccines (Basel). 2025. PMID: 40872874 Free PMC article.
-
Protective Immunity Induced by Virus-Like Particle Containing Merozoite Surface Protein 9 of Plasmodium berghei.Vaccines (Basel). 2020 Jul 30;8(3):428. doi: 10.3390/vaccines8030428. Vaccines (Basel). 2020. PMID: 32751598 Free PMC article.
-
Influenza Virus-Like Particles Presenting both Toxoplasma gondii ROP4 and ROP13 Enhance Protection against T. gondii Infection.Pharmaceutics. 2019 Jul 16;11(7):342. doi: 10.3390/pharmaceutics11070342. Pharmaceutics. 2019. PMID: 31315212 Free PMC article.
-
Recent progress in microneme-based vaccines development against Toxoplasma gondii.Clin Exp Vaccine Res. 2018 Jul;7(2):93-103. doi: 10.7774/cevr.2018.7.2.93. Epub 2018 Jul 31. Clin Exp Vaccine Res. 2018. PMID: 30112348 Free PMC article. Review.
-
Protection induced by malaria virus-like particles containing codon-optimized AMA-1 of Plasmodium berghei.Malar J. 2019 Dec 3;18(1):394. doi: 10.1186/s12936-019-3017-2. Malar J. 2019. PMID: 31796032 Free PMC article.
References
-
- Fayer R, Dubey JP, Lindsay DS. Zoonotic protozoa: from land to sea. Trends Parasitol. 2004;20: 531–536. doi: 10.1016/j.pt.2004.08.008 - DOI - PubMed
-
- Dubey J, Hill D, Jones J, Hightower A, Kirkland E, Roberts J, et al. Prevalence of viable Toxoplasma gondii in beef, chicken, and pork from retail meat stores in the United States: risk assessment to consumers. J Parasitol. 2005;91: 1082–1093. doi: 10.1645/GE-683.1 - DOI - PubMed
-
- Innes EA. Vaccination against Toxoplasma gondii: an increasing priority for collaborative research? 2010;9: 1117–1119. doi: 10.1586/erv.10.113 - DOI - PubMed
-
- Lee DH, Lee SH, Kim AR, Quan FS. Virus-Like Nanoparticle Vaccine Confers Protection against Toxoplasma gondii. 2016;11: e0161231 doi: 10.1371/journal.pone.0161231 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

