Pharmacogenetic variants in TPMT alter cellular responses to cisplatin in inner ear cell lines
- PMID: 28406961
- PMCID: PMC5391095
- DOI: 10.1371/journal.pone.0175711
Pharmacogenetic variants in TPMT alter cellular responses to cisplatin in inner ear cell lines
Abstract
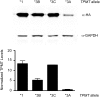
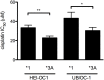
Cisplatin is a highly-effective and widely-used chemotherapeutic agent that causes ototoxicity in many patients. Pharmacogenomic studies of key genes controlling drug biotransformation identified variants in thiopurine methyltransferase (TPMT) as predictors of cisplatin-induced ototoxicity, although the mechanistic basis of this interaction has not been reported. Expression constructs of TPMT*3A, *3B and *3C variants were generated and monitored in cultured cells. Cellular TPMT*3A levels were detected at >20-fold lower amounts than the wild type confirming the unstable nature of this variant. The expression of wild type TPMT (TPMT*1) in two murine ear cell lines, HEI-OC1 and UB/OC-1, significantly mitigated their susceptibility to cisplatin toxicity. Cisplatin treatment induced Tlr4 gene expression in HEI-OC1 cells and this response was blunted by the expression of wild type TPMT but not TPMT*3A. In line with the significant mitigation of TPMT*1-expressing cells to cisplatin cytotoxicity, these findings demonstrate a drug-gene interaction between increased TPMT activity and decreased susceptibility to cisplatin-induced toxicity of inner ear cells.
Conflict of interest statement
Figures

Similar articles
-
Functional characterization of 23 allelic variants of thiopurine S-methyltransferase gene (TPMT*2 - *24).Pharmacogenet Genomics. 2008 Oct;18(10):887-93. doi: 10.1097/FPC.0b013e3283097328. Pharmacogenet Genomics. 2008. PMID: 18708949
-
Using HapMap tools in pharmacogenomic discovery: the thiopurine methyltransferase polymorphism.Clin Pharmacol Ther. 2007 May;81(5):729-34. doi: 10.1038/sj.clpt.6100135. Epub 2007 Feb 28. Clin Pharmacol Ther. 2007. PMID: 17329987
-
The role of inherited TPMT and COMT genetic variation in cisplatin-induced ototoxicity in children with cancer.Clin Pharmacol Ther. 2013 Aug;94(2):252-9. doi: 10.1038/clpt.2013.121. Epub 2013 Jun 11. Clin Pharmacol Ther. 2013. PMID: 23820299 Free PMC article.
-
Assessing cisplatin-induced ototoxicity and otoprotection in whole organ culture of the mouse inner ear in simulated microgravity.Toxicol Lett. 2014 Jun 16;227(3):203-12. doi: 10.1016/j.toxlet.2014.03.022. Epub 2014 Apr 5. Toxicol Lett. 2014. PMID: 24709139
-
Erdosteine protects HEI-OC1 auditory cells from cisplatin toxicity through suppression of inflammatory cytokines and induction of Nrf2 target proteins.Toxicol Appl Pharmacol. 2015 Oct 15;288(2):192-202. doi: 10.1016/j.taap.2015.07.014. Epub 2015 Jul 18. Toxicol Appl Pharmacol. 2015. PMID: 26193055
Cited by
-
The genetic vulnerability to cisplatin ototoxicity: a systematic review.Sci Rep. 2019 Mar 5;9(1):3455. doi: 10.1038/s41598-019-40138-z. Sci Rep. 2019. PMID: 30837596 Free PMC article.
-
Effects of subchronic inhalation exposure to an organophosphorus insecticide compound containing dichlorvos on wistar rats' otoacoustic emissions.Braz J Otorhinolaryngol. 2022 Jan-Feb;88(1):28-35. doi: 10.1016/j.bjorl.2020.04.005. Epub 2020 May 19. Braz J Otorhinolaryngol. 2022. PMID: 32532628 Free PMC article.
-
The Role of Genetic and Non-Genetic Factors in the Occurrence of Cisplatin-Associated Ototoxicity.Int J Mol Sci. 2025 May 16;26(10):4787. doi: 10.3390/ijms26104787. Int J Mol Sci. 2025. PMID: 40429930 Free PMC article. Review.
-
Detecting Novel Ototoxins and Potentiation of Ototoxicity by Disease Settings.Front Neurol. 2021 Aug 17;12:725566. doi: 10.3389/fneur.2021.725566. eCollection 2021. Front Neurol. 2021. PMID: 34489859 Free PMC article. Review.
References
-
- Estlin EJ, Veal GJ. Clinical and cellular pharmacology in relation to solid tumours of childhood. Cancer Treat Rev. 2003;29(4):253–73. - PubMed
-
- Brock PR, Knight KR, Freyer DR, Campbell KC, Steyger PS, Blakley BW, et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(19):2408–17. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

