A network of cis and trans interactions is required for ParB spreading
- PMID: 28407103
- PMCID: PMC5499601
- DOI: 10.1093/nar/gkx271
A network of cis and trans interactions is required for ParB spreading
Abstract
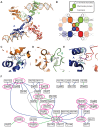
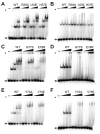
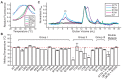
Most bacteria utilize the highly conserved parABS partitioning system in plasmid and chromosome segregation. This system depends on a DNA-binding protein ParB, which binds specifically to the centromere DNA sequence parS and to adjacent non-specific DNA over multiple kilobases in a phenomenon called spreading. Previous single-molecule experiments in combination with genetic, biochemical and computational studies have argued that ParB spreading requires cooperative interactions between ParB dimers including DNA bridging and possible nearest-neighbor interactions. A recent structure of a ParB homolog co-crystallized with parS revealed that ParB dimers tetramerize to form a higher order nucleoprotein complex. Using this structure as a guide, we systematically ablated a series of proposed intermolecular interactions in the Bacillus subtilis ParB (BsSpo0J) and characterized their effect on spreading using both in vivo and in vitro assays. In particular, we measured DNA compaction mediated by BsSpo0J using a recently developed single-molecule method to simultaneously visualize protein binding on single DNA molecules and changes in DNA conformation without protein labeling. Our results indicate that residues acting as hubs for multiple interactions frequently led to the most severe spreading defects when mutated, and that a network of both cis and trans interactions between ParB dimers is necessary for spreading.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



Similar articles
-
Permissive zones for the centromere-binding protein ParB on the Caulobacter crescentus chromosome.Nucleic Acids Res. 2018 Feb 16;46(3):1196-1209. doi: 10.1093/nar/gkx1192. Nucleic Acids Res. 2018. PMID: 29186514 Free PMC article.
-
Insights into ParB spreading from the complex structure of Spo0J and parS.Proc Natl Acad Sci U S A. 2015 May 26;112(21):6613-8. doi: 10.1073/pnas.1421927112. Epub 2015 May 11. Proc Natl Acad Sci U S A. 2015. PMID: 25964325 Free PMC article.
-
Phase-separated ParB enforces diverse DNA compaction modes and stabilizes the parS-centered partition complex.Nucleic Acids Res. 2024 Aug 12;52(14):8385-8398. doi: 10.1093/nar/gkae533. Nucleic Acids Res. 2024. PMID: 38908027 Free PMC article.
-
Bacterial chromosome segregation by the ParABS system.Open Biol. 2020 Jun;10(6):200097. doi: 10.1098/rsob.200097. Epub 2020 Jun 17. Open Biol. 2020. PMID: 32543349 Free PMC article. Review.
-
CTP switches in ParABS-mediated bacterial chromosome segregation and beyond.Curr Opin Microbiol. 2023 Jun;73:102289. doi: 10.1016/j.mib.2023.102289. Epub 2023 Mar 3. Curr Opin Microbiol. 2023. PMID: 36871427 Review.
Cited by
-
Compaction and control-the role of chromosome-organizing proteins in Streptomyces.FEMS Microbiol Rev. 2020 Nov 24;44(6):725-739. doi: 10.1093/femsre/fuaa028. FEMS Microbiol Rev. 2020. PMID: 32658291 Free PMC article. Review.
-
Dynamic ParB-DNA interactions initiate and maintain a partition condensate for bacterial chromosome segregation.Nucleic Acids Res. 2023 Nov 27;51(21):11856-11875. doi: 10.1093/nar/gkad868. Nucleic Acids Res. 2023. PMID: 37850647 Free PMC article.
-
Single-molecule DNA-flow stretching assay as a versatile hybrid tool for investigating DNA-protein interactions.BMB Rep. 2025 Jan;58(1):41-51. doi: 10.5483/BMBRep.2024-0177. BMB Rep. 2025. PMID: 39701027 Free PMC article. Review.
-
Permissive zones for the centromere-binding protein ParB on the Caulobacter crescentus chromosome.Nucleic Acids Res. 2018 Feb 16;46(3):1196-1209. doi: 10.1093/nar/gkx1192. Nucleic Acids Res. 2018. PMID: 29186514 Free PMC article.
-
Pseudomonas aeruginosa partitioning protein ParB acts as a nucleoid-associated protein binding to multiple copies of a parS-related motif.Nucleic Acids Res. 2018 May 18;46(9):4592-4606. doi: 10.1093/nar/gky257. Nucleic Acids Res. 2018. PMID: 29648658 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

