Autologous hematopoietic stem cell transplantation following high-dose chemotherapy for nonrhabdomyosarcoma soft tissue sarcomas
- PMID: 28407197
- PMCID: PMC6478255
- DOI: 10.1002/14651858.CD008216.pub5
Autologous hematopoietic stem cell transplantation following high-dose chemotherapy for nonrhabdomyosarcoma soft tissue sarcomas
Abstract
Background: Soft tissue sarcomas (STS) are a highly heterogeneous group of rare malignant solid tumors. Nonrhabdomyosarcoma soft tissue sarcomas (NRSTS) comprise all STS except rhabdomyosarcoma. In people with advanced local or metastatic disease, autologous hematopoietic stem cell transplantation (HSCT) applied after high-dose chemotherapy (HDCT) is a planned rescue therapy for HDCT-related severe hematologic toxicity. The rationale for this update is to determine whether any randomized controlled trials (RCTs) have been conducted and to clarify whether HDCT followed by autologous HSCT has a survival advantage.
Objectives: To assess the efficacy and safety of high-dose chemotherapy (HDCT) followed by autologous hematopoietic stem cell transplantation (HSCT) for all stages of nonrhabdomyosarcoma soft tissue sarcomas (NRSTS) in children and adults.
Search methods: For this update, we revised the search strategy to improve the precision and reduce the number of irrelevant hits. We searched the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8), PubMed from 2012 to 6 September 2016, and Embase from 2012 to 26 September 2016. We searched online trial registries and congress proceedings from 2012 to 26 September 2016.
Selection criteria: Terms representing STS and autologous HSCT were required in the title or abstract. We restricted the study design to RCTs. We included studies if at least 80% of participants had a diagnosis listed in any version of the World Health Organization (WHO) classification and classified as malignant. The search included children and adults with no age limits.
Data collection and analysis: We used standard methodologic procedures expected by Cochrane. The primary outcomes were overall survival and treatment-related mortality.
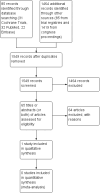
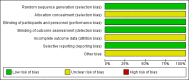
Main results: We identified 1549 records; 85 items from electronic databases, 45 from study registries, and 1419 from congress proceedings. The revised search strategy did not identify any additional RCTs. In the previous version of the review, we identified one RCT comparing HDCT followed by autologous HSCT versus standard-dose chemotherapy (SDCT). The trial randomized 87 participants who were considerably heterogeneous with respect to 19 different tumor entities. The data from 83 participants were available for analysis.In the single included trial, overall survival at three years was 32.7% in the HDCT arm versus 49.4% in the SDCT arm and there was no difference between the treatment groups (hazard ratio (HR) 1.26, 95% confidence interval (CI) 0.70 to 2.29, P = 0.44; 1 study, 83 participants; high quality evidence). In a subgroup of participants who had a complete response before HDCT, overall survival was higher in both treatment groups and overall survival at three years was 42.8% in the HDCT arm versus 83.9% in the SDCT arm and favored the SDCT group (HR 2.92, 95% CI 1.1 to 7.6, P = 0.028; 1 study, 39 participants).In the single included trial, the authors reported one treatment-related leukemia death two years after HDCT. They also evaluated severe adverse events WHO grade 3 to 4 in 22 participants in the HDCT arm and in 51 participants in the SDCT arm. The authors reported 11 events concerning digestive-, infection-, pain-, or asthenia-related toxicity in the HDCT arm and one event in the SDCT arm (moderate quality evidence). The development of secondary neoplasia was not addressed. We judged the study to have an overall unclear risk of bias as three of seven items had unclear and four items had low risk of bias. For GRADE, we judged three items as high quality and three items were not reported.
Authors' conclusions: The limited data of a single RCT with an unclear risk of bias and moderate to high quality evidence showed no survival advantage for HDCT. If this treatment is offered it should only be given after careful consideration on an individual person basis and possibly only as part of a well-designed RCT.
Conflict of interest statement
FP: none known.
HE: none known.
LAS: none known.
Figures
Update of
-
Autologous hematopoietic stem cell transplantation following high dose chemotherapy for non-rhabdomyosarcoma soft tissue sarcomas.Cochrane Database Syst Rev. 2013 Aug 7;2013(8):CD008216. doi: 10.1002/14651858.CD008216.pub4. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2017 Apr 13;4:CD008216. doi: 10.1002/14651858.CD008216.pub5. PMID: 23925699 Free PMC article. Updated.
Similar articles
-
Autologous hematopoietic stem cell transplantation following high dose chemotherapy for non-rhabdomyosarcoma soft tissue sarcomas.Cochrane Database Syst Rev. 2013 Aug 7;2013(8):CD008216. doi: 10.1002/14651858.CD008216.pub4. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2017 Apr 13;4:CD008216. doi: 10.1002/14651858.CD008216.pub5. PMID: 23925699 Free PMC article. Updated.
-
Autologous hematopoietic stem cell transplantation following high-dose chemotherapy for non-rhabdomyosarcoma soft tissue sarcomas.Cochrane Database Syst Rev. 2011 Feb 16;(2):CD008216. doi: 10.1002/14651858.CD008216.pub3. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2013 Aug 07;(8):CD008216. doi: 10.1002/14651858.CD008216.pub4. PMID: 21328307 Updated.
-
Retinoic acid postconsolidation therapy for high-risk neuroblastoma patients treated with autologous haematopoietic stem cell transplantation.Cochrane Database Syst Rev. 2017 Aug 25;8(8):CD010685. doi: 10.1002/14651858.CD010685.pub3. Cochrane Database Syst Rev. 2017. PMID: 28840597 Free PMC article.
-
High-dose chemotherapy and autologous haematopoietic stem cell rescue for children with high-risk neuroblastoma.Cochrane Database Syst Rev. 2015 Oct 5;2015(10):CD006301. doi: 10.1002/14651858.CD006301.pub4. Cochrane Database Syst Rev. 2015. PMID: 26436598 Free PMC article.
-
High-dose chemotherapy and autologous haematopoietic stem cell rescue for children with high-risk neuroblastoma.Cochrane Database Syst Rev. 2013 Aug 22;(8):CD006301. doi: 10.1002/14651858.CD006301.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2015 Oct 05;(10):CD006301. doi: 10.1002/14651858.CD006301.pub4. PMID: 23970444 Updated.
Cited by
-
Antiemetic Prophylaxis with Fosaprepitant and 5-HT3-Receptor Antagonists in Pediatric Patients Undergoing Autologous Hematopoietic Stem Cell Transplantation.Drug Des Devel Ther. 2020 Sep 25;14:3915-3927. doi: 10.2147/DDDT.S260887. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33061297 Free PMC article.
-
Haematopoietic stem cell transplantation in adult soft-tissue sarcoma: an analysis from the European Society for Blood and Marrow Transplantation.ESMO Open. 2020 Oct;5(5):e000860. doi: 10.1136/esmoopen-2020-000860. ESMO Open. 2020. PMID: 33097652 Free PMC article.
-
Autologous Stem-Cell Transplantation for High-Risk Neuroblastoma: Historical and Critical Review.Cancers (Basel). 2022 May 24;14(11):2572. doi: 10.3390/cancers14112572. Cancers (Basel). 2022. PMID: 35681553 Free PMC article. Review.
References
References to studies included in this review
Bui‐Nguyen 2012 {published data only}
-
- Binh NN, Chevreau C, Penel N, Bay J, Coindre J, Mathoulin‐Pelissier S, et al. Consolidation with high‐dose chemotherapy for responding patients to standard chemotherapy in advanced, metastatic soft tissue sarcoma (STS): a randomized trial from FNCLCC‐French Sarcoma Group. Journal of Clinical Oncology 2009;27(15 Suppl):10505.
-
- Bui‐Nguyen B, Ray‐Coquard I, Chevreau C, Penel N, Bay JO, Coindre JM, et al. High‐dose chemotherapy consolidation for chemosensitive advanced soft tissue sarcoma patients: an open‐label, randomized controlled trial. Annals of Oncology 2012;23(3):777‐84. - PubMed
References to studies excluded from this review
Araki 2016 {published data only}
-
- Araki N, Takahashi S, Sugiura H, Ueda T, Yonemoto T, Takahashi M, et al. Retrospective inter‐ and intra‐patient evaluation of trabectedin after best supportive care for patients with advanced translocation‐related sarcoma after failure of standard chemotherapy. European Journal of Cancer 2016;56:122‐30. - PubMed
Benesch 2014 {published data only}
-
- Benesch M, Bartelheim K, Fleischhack G, Gruhn B, Schlegel PG, Witt O, et al. High‐dose chemotherapy (HDCT) with auto‐SCT in children with atypical teratoid/rhabdoid tumors (AT/RT): a report from the European Rhabdoid Registry (EU‐RHAB). Bone Marrow Transplantation 2014;49(3):370‐5. - PubMed
Brana 2014 {published data only}
-
- Brana I, Berger R, Golan T, Haluska P, Edenfield J, Fiorica J, et al. A parallel‐arm phase I trial of the humanised anti‐IGF‐1R antibody dalotuzumab in combination with the AKT inhibitor MK‐2206, the mTOR inhibitor ridaforolimus, or the NOTCH inhibitor MK‐0752, in patients with advanced solid tumours. British Journal of Cancer 2014;111(10):1932‐44. - PMC - PubMed
Calabro 2015 {published data only}
-
- Calabro L, Morra A, Fonsatti E, Cutaia O, Fazio C, Annesi D, et al. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy‐resistant malignant mesothelioma: an open‐label, single‐arm, phase 2 study. Lancet Respiratory Medicine 2015;3(4):301‐9. - PubMed
Cohen 2012 {published data only}
Conter 2013 {published data only}
-
- Conter HJ. Ten‐year follow‐up of patients (pts) with metastatic renal cell carcinoma (mRCC) treated with interferon alfa‐2b (IFN) as first‐line therapy: results from a randomized trial. Journal of Clinical Oncology 2013;31(Suppl 6):Abstr 365.
Czarnecka 2014 {published data only}
-
- Czarnecka AM, Szczylik C, Rini B. The use of sunitinib in renal cell carcinoma: where are we now?. Expert Review of Anticancer Therapy 2014;14(9):983‐99. - PubMed
Davis 2015 {published data only}
-
- Davis EJ, Chugh R, Zhao L, Lucas DR, Biermann JS, Zalupski MM, et al. A randomised, open‐label, phase II study of neo/adjuvant doxorubicin and ifosfamide versus gemcitabine and docetaxel in patients with localised, high‐risk, soft tissue sarcoma. European Journal of Cancer 2015;51(13):1794‐802. - PubMed
Delannes 2013 {published data only}
-
- Delannes M, Thomas L, Brun T, David I, Ducassou A. Brachytherapy for extremity soft tissue sarcomas [Curietherapie des sarcomes des tissus mous des membres]. Cancer Radiotherapie 2013;17(2):151‐4. - PubMed
Demetri 2016 {published data only}
-
- Demetri GD, Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. Journal of Clinical Oncology 2016;34(8):786‐93. - PMC - PubMed
Diez‐Tejedor 2014 {published data only}
-
- Diez‐Tejedor E, Gutierrez‐Fernandez M, Martinez‐Sanchez P, Rodriguez‐Frutos B, Ruiz‐Ares G, Lara ML, et al. Reparative therapy for acute ischemic stroke with allogeneic mesenchymal stem cells from adipose tissue: a safety assessment: a phase II randomized, double‐blind, placebo‐controlled, single‐center, pilot clinical trial. Journal of Stroke and Cerebrovascular Diseases 2014;23(10):2694‐700. - PubMed
Drabko 2012 {published data only}
-
- Drabko K, Raciborska A, Bilska K, Styczynski J, Ussowicz M, Choma M, et al. Consolidation of first‐line therapy with busulphan and melphalan, and autologous stem cell rescue in children with Ewing's sarcoma. Bone Marrow Transplantation 2012;47(12):1530‐4. - PubMed
Ehlert 2012 {published data only}
-
- Ehlert K, Rossig C, Groll A, Waeltermann M, Froehlich B, Juergens H. Toxicity of treosulfan in paediatric high‐dose chemotherapy regimens. Bone Marrow Transplantation 2012;47:S380.
Engelhard 2013 {published data only}
-
- Engelhard M, Allgauer M, Amela‐Neuschwander S, Brand HU, Brandes A, Bucker R, et al. Follicular lymphoma: final results of the randomized evaluation of curative radiotherapy in limited stage nodal disease. Strahlentherapie und Onkologie 2013;189(Suppl 1):1‐152.
Friedman 2014 {published data only}
-
- Friedman DL, Chen L, Wolden S, Buxton A, McCarten K, FitzGerald TJ, et al. Dose‐intensive response‐based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate‐risk Hodgkin lymphoma: a report from the Children's Oncology Group Study AHOD0031. Journal of Clinical Oncology 2014;32(32):3651‐8. - PMC - PubMed
Froeb 2012 {published data only}
-
- Froeb D, Ranft A, Boelling T, Paulussen M, Klco‐Brosius S, Jurgens H, et al. Ewing sarcoma of the hand or foot. Klinische Padiatrie 2012;224(6):348‐52. - PubMed
Gaspar 2012 {published data only}
-
- Gaspar N, Rey A, Berard PM, Michon J, Gentet JC, Tabone MD, et al. Risk adapted chemotherapy for localised Ewing's sarcoma of bone: the French EW93 study. European Journal of Cancer 2012;48(9):1376‐85. - PubMed
Grignani 2015 {published data only}
-
- Grignani G, Palmerini E, Ferraresi V, D'Ambrosio L, Bertulli R, Asaftei SD, et al. Sorafenib and everolimus for patients with unresectable high‐grade osteosarcoma progressing after standard treatment: a non‐randomised phase 2 clinical trial. Lancet Oncology 2015;16(1):98‐107. - PubMed
Gronchi 2012 {published data only}
-
- Gronchi A, Frustaci S, Mercuri M, Martin J, Lopez‐Pousa A, Verderio P, et al. Short, full‐dose adjuvant chemotherapy in high‐risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. Journal of Clinical Oncology 2012;30(8):850‐6. - PubMed
Halland 2012 {published data only}
-
- Halland AM. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate at one year ‐ the LITHE study the use of radiographs as a measure of structural damage. European Musculoskeletal Review 2012;7(4):eprint.
Hartmann 2013 {published data only}
-
- Hartmann JT, Horger M, Kluba T, Konigsrainer A, Zwart P, Weyhern CH, et al. A non‐comparative phase II study of dose intensive chemotherapy with doxorubicin and ifosfamide followed by high dose ICE consolidation with PBSCT in non‐resectable, high grade, adult type soft tissue sarcomas. Investigational New Drugs 2013;31(6):1592‐601. - PubMed
Hensley 2013 {published data only}
-
- Hensley ML, Wathen JK, Maki RG, Araujo DM, Sutton G, Priebat DA, et al. Adjuvant therapy for high‐grade, uterus‐limited leiomyosarcoma: results of a phase 2 trial (SARC 005). Cancer 2013;119(8):1555‐61. - PubMed
Infante 2015 {published data only}
-
- Infante JR, Ramanathan RK, George D, Tan E, Quinlan M, Liu A, et al. A randomized, crossover phase 1 study to assess the effects of formulation (capsule vs tablet) and meal consumption on the bioavailability of dovitinib (TKI258). Cancer Chemotherapy and Pharmacology 2015;75(4):729‐37. - PubMed
Ishida 2014 {published data only}
-
- Ishida Y, Qiu D, Maeda M, Fujimoto J, Kigasawa H, Kobayashi R, et al. Secondary cancers after cancer diagnosis in childhood: a hospital‐based retrospective cohort study in Japan. Pediatric Blood and Cancer 2014;61:S198. - PubMed
Ishida 2016 {published data only}
-
- Ishida Y, Qiu D, Maeda M, Fujimoto J, Kigasawa H, Kobayashi R, et al. Secondary cancers after a childhood cancer diagnosis: a nationwide hospital‐based retrospective cohort study in Japan. International Journal of Clinical Oncology 2016;21(3):506‐16. - PubMed
Joensuu 2012 {published data only}
-
- Joensuu H. Adjuvant therapy for high‐risk gastrointestinal stromal tumour: considerations for optimal management. Drugs 2012;72(15):1953‐63. - PubMed
Judson 2014 {published data only}
-
- Judson I, Verweij J, Gelderblom H, Hartmann JT, Schoffski P, Blay JY, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first‐line treatment of advanced or metastatic soft‐tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncology 2014;15(4):415‐23. - PubMed
Kawai 2015 {published data only}
-
- Kawai A, Araki N, Sugiura H, Ueda T, Yonemoto T, Takahashi M, et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation‐related sarcoma: a randomised, open‐label, phase 2 study. Lancet Oncology 2015;16(4):406‐16. - PubMed
Khmelevsky 2015 {published data only}
-
- Khmelevsky EV, Bychkova NM. Radiosensitivity of bone metastases from tumors in different primary sites [Russian]. Voprosy Onkologii 2015;61(1):77‐84. - PubMed
Lanza 2015 {published data only}
-
- Lanza F, Pedrazzoli P, Ravelli A, Brambilla P. Hematopoietic stem cell transplantation in solid tumors. 41st Annual Meeting of the European Society for Blood and Marrow Transplantation, EBMT 2015. Istanbul Turkey. Bone Marrow Transplantation 2015;50:S114.
Laws 2014 {published data only}
-
- Laws DA, Kraft AS, Leddy LR, LaRue AC. The role of circulating fibroblast precursors in promoting metastatic sarcoma. Cancer Research 2014;75(1 Suppl 1):A46.
Le Deley 2014 {published data only}
-
- Deley MC, Paulussen M, Lewis I, Brennan B, Ranft A, Whelan J, et al. Cyclophosphamide compared with ifosfamide in consolidation treatment of standard‐risk Ewing sarcoma: results of the randomized noninferiority Euro‐EWING99‐R1 trial. Journal of Clinical Oncology 2014;32(23):2440‐8. - PubMed
Liu 2012 {published data only}
-
- Liu CJ, Chen KW, Hu YW, Hong YC, Huang YC, Chiou TJ, et al. Hematopoietic stem cell transplantation and secondary primary malignancies in Taiwan. Blood 2012;120(21):4219.
Liu 2013a {published data only}
-
- Liu SL, Chen G, Zhao YP, Wu WM, Zhang TP. Optimized dose of imatinib for treatment of gastrointestinal stromal tumors: a meta‐analysis. Journal of Digestive Diseases 2013;14(1):16‐21. - PubMed
Liu 2013b {published data only}
-
- Liu J, Yu H, Shang Q, Yan C, Jiang P, Wang X. The effects of low‐dose splenic irradiation and radiotherapy on immune system of patients with locally advanced non‐small cell lung cancer. Chinese‐German Journal of Clinical Oncology 2013;12(2):51‐5.
Maher 2014 {published data only}
-
- Maher O, Cooper L, Tarek N, Worth LL, Petropoulis D, Lee DA, et al. Successful treatment for secondary AML and MDS following myeloablative hematopoetic stem cell transplantation for children, adolescents and young adults. Bone Marrow Transplantation 2014;49:S376.
Merker 2015 {published data only}
-
- Merker M, Rettinger E, Oelsner S, Pfirrmann V, Ullrich E, Wels W, et al. ErbB2‐chimeric antigen receptor (CAR) modified cytokine induced killer (CIK) cell intervention for refractory solid tumors after allogeneic stem cell transplantation. Bone Marrow Transplantation 2015;50:S93.
Meyers 2015 {published data only}
-
- Meyers PA. Systemic therapy for osteosarcoma and Ewing sarcoma. American Society of Clinical Oncology Educational Book 2015:e644‐7. - PubMed
Munir 2016 {published data only}
-
- Munir T, Cohen D, Pocock C, Rawstron A, Collet L, McParland L, et al. Physical examination and MRD assessment rather than CT‐scans is effective to assess responses in patients treated with fludarabine‐based chemoimmunotherapy. Analysis of patients in the UK NCRI ADMIRE and ARCTIC trials. Leukemia & Lymphoma 2015;56:26‐7.
Narumi 2012 {published data only}
-
- Narumi K, Udagawa T, Suzuki K, Aida K, Makimoto A, Aoki K. Syngeneic hematopoietic stem cell transplantation enhances the antitumor immunity of intratumoral type I interferon gene transfer for sarcoma. Molecular Therapy 2012;20:S263. - PubMed
Oberlin 2012 {published data only}
-
- Oberlin O, Rey A, Sanchez de Toledo J, Martelli H, Jenney ME, Scopinaro M, et al. Randomized comparison of intensified six‐drug versus standard three‐drug chemotherapy for high‐risk nonmetastatic rhabdomyosarcoma and other chemotherapy‐sensitive childhood soft tissue sarcomas: long‐term results from the International Society of Pediatric Oncology MMT95 study. Journal of Clinical Oncology 2012;30(20):2457‐65. - PubMed
Palassini 2015 {published data only}
-
- Palassini E, Ferrari S, Verderio P, Paoli A, Martin Broto J, Quagliuolo V, et al. Feasibility of preoperative chemotherapy with or without radiation therapy in localized soft tissue sarcomas of limbs and superficial trunk in the Italian Sarcoma Group/Grupo Espanol de Investigacion en Sarcomas randomized clinical trial: three versus five cycles of full‐dose epirubicin plus ifosfamide. Journal of Clinical Oncology 2015;33(31):3628‐34. - PubMed
Pavlyk 2013 {published data only}
-
- Pavlyk S, Shaida E, Grigoriy K. Metronomic application of cyclophosphamide and vinorelbine for treatment of soft‐tissue sarcoma in children. Pediatric Blood and Cancer 2013;60:37.
Peinemann 2013 {published data only}
Peinemann 2014 {published data only}
Porta 2014 {published data only}
-
- Porta C, Giglione P, Lombardo F, Paglino C. First‐line medical therapy in advanced metastatic RCC (conference abstract). Anticancer Research 2014;34(10):6117‐8.
Rettinger 2012a {published data only}
Rettinger 2012b {published data only}
-
- Rettinger E, Meyer V, Kreyenberg H, Kuci S, Willasch A, Fulda S, et al. Clinical scale generation and functional assessment of cytokine‐induced killer cells against acute leukaemia and soft tissue sarcoma. Bone Marrow Transplantation 2012;47:S361.
Schmidinger 2012 {published data only}
-
- Schmidinger M, Szczylik C, Sternberg CN, Kania M, Kelly CS, Decker R, et al. Dose escalation and pharmacokinetics study of enzastaurin and sunitinib versus placebo and sunitinib in patients with metastatic renal cell carcinoma. American Journal of Clinical Oncology 2012;35(5):493‐7. - PubMed
Schoffski 2016 {published data only}
-
- Schoffski P, Chawla S, Maki RG, Italiano A, Gelderblom H, Choy E, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open‐label, multicentre, phase 3 trial. Lancet 2016;387(10028):1629‐37. - PubMed
Shvarova 2012 {published data only}
-
- Shvarova A, Ivanova N, Dolgopolov I. The survivorship of children with primary metastatic bone and soft tissue sarcomas. 2012 International MASCC/ISOO Symposium. New York City, NY. Supportive Care in Cancer 2012;20:S86‐7.
Smolen 2014 {published data only}
-
- Smolen JS, Weinblatt ME, Sheng S, Zhuang Y, Hsu B. Sirukumab, a human anti‐interleukin‐6 monoclonal antibody: a randomised, 2‐part (proof‐of‐concept and dose‐finding), phase II study in patients with active rheumatoid arthritis despite methotrexate therapy. Annals of the Rheumatic Diseases 2014;73(9):1616‐25. - PMC - PubMed
Stahel 2015 {published data only}
-
- Stahel RA, Riesterer O, Xyrafas A, Opitz I, Beyeler M, Ochsenbein A, et al. Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAKK 17/04): a randomised, international, multicentre phase 2 trial. Lancet Oncology 2015;16(16):1651‐8. - PubMed
Teichert von Luettichau 2014 {published data only}
-
- Teichert von Luettichau I, Salat C, Wawer A, Noessner E, Eckl J, Multhoff G, et al. Targeting the graft‐versus‐tumor effect of DLI by regional hyperthermia to the tumor compartment. Bone Marrow Transplantation 2014;49:S499.
Teppo 2016 {published data only}
-
- Teppo E, Penttinen J, Myohanen O, Vettenranta K, Lohi O. Single‐centre study reports a 84% five‐year overall survival rate for paediatric solid tumours. Acta Paediatrica 2016;105(8):952‐8. - PubMed
Uehara 2015 {published data only}
-
- Uehara S, Oue T, Nakahata K, Nara K, Ueno T, Owari M, et al. Perioperative management after high‐dose chemotherapy with autologous or allogeneic hematopoietic stem cell transplantation for pediatric solid tumors. European Journal of Pediatric Surgery 2015;25(1):118‐22. - PubMed
Vecsei 2014 {published data only}
-
- Vecsei A, Engert Z, Bardi E. Primary inoperable pelvic embryonal rhabdomyosarcoma became operable and curable due to complex therapy. EAU 14th Central European Meeting, CEM 2014. Cracow Poland. European Urology, Supplements 2014;13(6):e1365.
Verweij 2013 {published data only}
-
- Verweij J, Sleijfer S. Pazopanib, a new therapy for metastatic soft tissue sarcoma. Expert Opinion on Pharmacotherapy 2013;14(7):929‐35. - PubMed
Woll 2012 {published data only}
-
- Woll PJ, Reichardt P, Cesne A, Bonvalot S, Azzarelli A, Hoekstra HJ, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft‐tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncology 2012;13(10):1045‐54. - PubMed
Womer 2012 {published data only}
Yamada 2012 {published data only}
-
- Yamada K, Inoue M, Kondo O, Sato M, Sawada A, Yasui M, et al. Issues associated with cancer in adolescents and young adults. 10th Annual Meeting of the Japanese Society of Medical Oncology. Osaka Japan. Annals of Oncology 2012;23:xi167.
Yuan 2015 {published data only}
-
- Yuan J, Liu T, Zhang X, Si Y, Ye Y, Zhao C, et al. Intensive versus conventional glycemic control in patients with diabetes during enteral nutrition after gastrectomy. Journal of Gastrointestinal Surgery 2015;19(8):1553‐8. - PubMed
Zhang 2013 {published data only}
-
- Zhang X, Wang Z, Wang Z, Zhang Y, Jia Q, Wu L, et al. Impact of acetylsalicylic acid on tumor angiogenesis and lymphangiogenesis through inhibition of VEGF signaling in a murine sarcoma model. Oncology Reports 2013;29(5):1907‐13. - PubMed
Zhou 2013 {published data only}
-
- Zhou A, Zhang W, Chang C, Chen X, Zhong D, Qin Q, et al. Phase I study of the safety, pharmacokinetics and antitumor activity of famitinib. Cancer Chemotherapy and Pharmacology 2013;72(5):1043‐53. - PubMed
Zhu 2014 {published data only}
-
- Zhu HD, Guo JH, Mao AW, Lv WF, Ji JS, Wang WH, et al. Conventional stents versus stents loaded with (125)iodine seeds for the treatment of unresectable oesophageal cancer: a multicentre, randomised phase 3 trial. Lancet Oncology 2014;15(6):612‐9. - PubMed
Additional references
ACS 2016
-
- American Cancer Society. Survival by stage of soft tissue sarcoma. www.cancer.org/cancer/sarcoma‐adultsofttissuecancer/detailedguide/sarcom... (accessed prior to 28 March 2017).
Admiraal 2010
AJCC 2016
-
- American Joint Committee on Cancer. Soft tissue sarcoma. In: Amin MB, Edge S, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. editor(s). AJCC Cancer Staging Manual. 8th Edition. New York: Springer, 2016.
ASCO Meetings 2012 to 2016
-
- Meeting Library Search Abstracts 2012 to 2016. American Society of Clinical Oncology (ASCO), Alexandria, Virginia, U.S.A. 2016, issue http://meetinglibrary.asco.org/abstracts.
ASH Meetings 2013 to 2015
-
- ASH Annual Meeting Abstracts 2013 to 2015. American Society of Hematology (ASH), Washington, District of Columbia, U.S.A. 2016, issue http://www.bloodjournal.org/page/ash‐annual‐meeting‐abstracts.
Banna 2007
-
- Banna GL, Simonelli M, Santoro A. High‐dose chemotherapy followed by autologous hematopoietic stem‐cell transplantation for the treatment of solid tumors in adults: a critical review. Current Stem Cell Research and Therapy 2007;2(1):65‐82. - PubMed
Blay 2000
-
- Blay JY, Bouhour D, Ray‐Coquard I, Dumontet C, Philip T, Biron P. High‐dose chemotherapy with autologous hematopoietic stem‐cell transplantation for advanced soft tissue sarcoma in adults. Journal of Clinical Oncology 2000;18(21):3643‐50. - PubMed
BMT Tandem Meeting 2012
-
- Abstracts from the 2012 BMT Tandem Meetings. Biology of Blood and Marrow Transplantation. 2012; Vol. 18, issue 2 Suppl:S201‐406.
BMT Tandem Meeting 2013
-
- Abstracts from the 2013 BMT Tandem Meetings. Biology of Blood and Marrow Transplantation. 2013; Vol. 19, issue 2 Suppl:S109‐392.
BMT Tandem Meeting 2014
-
- Abstracts from the 2014 BMT Tandem Meetings. Biology of Blood and Marrow Transplantation. 2014; Vol. 20, issue 2 Suppl:S1‐326. - PubMed
BMT Tandem Meeting 2015
-
- Abstracts from the 2015 BMT Tandem Meetings. Biology of Blood and Marrow Transplantation. 2015; Vol. 21, issue 2 Suppl:S1‐410. - PubMed
BMT Tandem Meeting 2016
-
- Abstracts from the 2016 BMT Tandem Meetings. Biology of Blood and Marrow Transplantation. 2016; Vol. 22, issue 3 Suppl:S1‐498. - PubMed
Bonnet 1997
-
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Medicine 1997;3(7):730‐7. - PubMed
Carvajal 2005
-
- Carvajal R, Meyers P. Ewing's sarcoma and primitive neuroectodermal family of tumors. Hematology/Oncology Clinics of North America 2005;19(3):501‐25. - PubMed
Clark 2005
-
- Clark MA, Fisher C, Judson I, Thomas JM. Soft‐tissue sarcomas in adults. New England Journal of Medicine 2005;353(7):701‐11. - PubMed
ClinicalTrials.gov Studies 2016
-
- U.S. National Library of Medicine (NLM), Bethesda, Maryland, U.S.A. ClinicalTrials.gov. clinicaltrials.gov (accessed 26 September 2016).
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Dileo 2005
-
- Dileo P, Demetri GD. Update on new diagnostic and therapeutic approaches for sarcomas. Clinical Advances in Hematology & Oncology 2005;3(10):781‐91. - PubMed
Dumontet 1992
-
- Dumontet C, Biron P, Bouffet E, Blay JY, Meckenstock R, Chauvin F, et al. High dose chemotherapy with ABMT in soft tissue sarcomas: a report of 22 cases. Bone Marrow Transplantation 1992;10(5):405‐8. - PubMed
EBMT Meeting 2014
-
- 40th Annual Meeting of the European Group for Blood and Marrow Transplantation, Milan, Italy, 30 March ‐ 2 April 2014. Bone Marrow Transplantation. 2014; Vol. 49:S1. - PubMed
EBMT Meeting 2015
-
- 41st Annual Meeting of the European Society for Blood and Marrow Transplantation, Istanbul, Turkey, 22‐25 March 2015. Bone Marrow Transplantation. 2015; Vol. 50:S1.
EBMT Meeting 2016
-
- 42nd Annual Meeting of the European Society for Blood and Marrow Transplantation Valencia, Spain 3‐6 April 2016. Bone Marrow Transplantation. 2016; Vol. 51:S1.
EBMT Studies 2016
-
- European Society for Blood and Marrow Transplantation (EBMT), Leiden, The Netherlands. EBMT Studies Current Research List. www.ebmt.org/Contents/Research/EBMTStudies/CurrentResearch/Pages/Current..., (accessed prior to 28 March 2017).
Ek 2006
-
- Ek ETH, Choong PFM. The role of high‐dose therapy and autologous stem cell transplantation for pediatric bone and soft tissue sarcomas. Expert Review of Anticancer Therapy 2006;6(2):225‐37. - PubMed
Elias 1998
-
- Elias AD. High‐dose therapy for adult soft tissue sarcoma: dose response and survival. Seminars in Oncology 1998;25(2 Suppl 4):19‐23. - PubMed
ESMO 2014
-
- European Society for Medical Oncology/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 2014;25 Suppl 3:iii102‐12. - PubMed
FDA 2015
-
- Kassim SY. Warning letter. Reference: 15‐HFD‐45‐05‐01. www.fda.gov/ICECI/EnforcementActions/WarningLetters/2015/ucm452031.htm (accessed prior to 28 March 2017).
Fletcher 2002
-
- Fletcher CDM, Rydholm A, Singer S, Sundaram M, Coindre JM. Soft tissue tumours: WHO classification, epidemiology, clinical features, histopathological typing and grading. In: Fletcher CDM, Unni KK, Mertens F editor(s). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon: International Agency for Research on Cancer, 2002:12‐8.
Fletcher 2013
-
- Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone. 4th Edition. Lyon: International Agency for Research on Cancer, 2013.
Google Translate 2016 [Computer program]
-
- Google, Inc. Google Translate. Mountain View: Google, Inc, 2016; translate.google.com/.
GRADEpro 2014 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Gurney 1997
-
- Gurney JG, Young JL, Roffers SD, Smith MA, Bunin GR. Soft tissue sarcomas. In: Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, et al. editor(s). Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program 1975‐1995 (SEER Pediatric Monograph) NIH Pub. No. 99‐4649. Bethesda: National Cancer Institute, 1997:111‐24.
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Howlader 2016
-
- Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al. SEER Cancer Statistics Review, 1975‐2013. seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER website, April 2016 (accessed prior to 28 March 2017).
ICTRP Studies 2016
-
- International Clinical Trials Registry Platform (ICTRP) Search Portal. World Health Organization 2016, issue http://apps.who.int/trialsearch/.
Kasper 2005
-
- Kasper B, Ho AD, Egerer G. Is there an indication for high‐dose chemotherapy in the treatment of bone and soft‐tissue sarcoma?. Oncology 2005;68(2‐3):15‐21. - PubMed
Kasper 2007
-
- Kasper B, Dietrich S, Mechtersheimer G, Ho AD, Egerer G. Large institutional experience with dose‐intensive chemotherapy and stem cell support in the management of sarcoma patients. Oncology 2007;73(1‐2):58‐64. - PubMed
Kotilingam 2006
-
- Kotilingam D, Lev DC, Lazar AJ, Pollock RE. Staging soft tissue sarcoma: evolution and change. CA: A Cancer Journal for Clinicians 2006;56(5):282‐91. - PubMed
Ladenstein 1997
-
- Ladenstein R, Philip T, Gardner H. Autologous stem cell transplantation for solid tumors in children. Current Opinion in Pediatrics 1997;9(1):55‐69. - PubMed
Miller 1995
-
- Miller RW, Young JL Jr, Novakovic B. Childhood cancer. Cancer 1995;75(1 Suppl):395‐405. - PubMed
Moher 2009
NCI CTCAE 2016
-
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) and Common Toxicity Criteria (CTC). ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed 2 June 2016).
NCI snapshot 2014
-
- National Cancer Institute. A snapshot of sarcoma: incidence and mortality. cancer.gov/research/progress/snapshots/sarcoma (accessed 2 June 2016).
NCI staging 2015
-
- National Cancer Institute. Cancer staging. cancer.gov/cancertopics/factsheet/detection/staging (accessed 2 June 2016).
Passweg 2016
Pinkerton 1986
-
- Pinkerton R, Philip T, Bouffet E, Lashford L, Kemshead J. Autologous bone marrow transplantation in paediatric solid tumours. Clinics in Haematology 1986;15(1):187‐203. - PubMed
Reichardt 2002
-
- Reichardt P. High‐dose chemotherapy in adult soft tissue sarcoma. Critical Reviews in Oncology/Hematology 2002;41(2):157‐67. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riedel 2012
Rosti 2002
-
- Rosti G, Ferrante P, Ledermann J, Leyvraz S, Ladenstein R, Koscielniak E, et al. High‐dose chemotherapy for solid tumors: results of the EBMT. Critical Reviews in Oncology/Hematology 2002;41(2):129‐40. - PubMed
Sanchez‐Garcia 2007
-
- Sanchez‐Garcia I, Vicente‐Duenas C, Cobaleda C. The theoretical basis of cancer‐stem‐cell‐based therapeutics of cancer: can it be put into practice?. BioEssays 2007;29(12):1269‐80. - PubMed
Schlemmer 2006
-
- Schlemmer M, Wendtner CM, Falk M, Abdel‐Rahman S, Licht T, Baumert J, et al. Efficacy of consolidation high‐dose chemotherapy with ifosfamide, carboplatin and etoposide (HD‐ICE) followed by autologous peripheral blood stem cell rescue in chemosensitive patients with metastatic soft tissue sarcomas. Oncology 2006;71(1‐2):32‐9. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Seeger 1991
-
- Seeger RC, Reynolds CP. Treatment of high‐risk solid tumors of childhood with intensive therapy and autologous bone marrow transplantation. Pediatric Clinics of North America 1991;38(2):393‐424. - PubMed
SIOP Meeting 2012
-
- International Society of Paediatric Oncology. 44th Congress of the International Society of Paediatric Oncology (SIOP) 2012 International Conference, 2012 Oct 5‐8; London. Pediatric Blood & Cancer 2012;59(6):965‐1152. - PubMed
SIOP Meeting 2013
-
- International Society of Paediatric Oncology. 45th Congress of the International Society of Paediatric Oncology (SIOP) 2013 International Conference, 2013 Sept 25‐28; Hong Kong, China. Pediatric Blood & Cancer 2013;60(Suppl 3):S2‐264. - PubMed
SIOP Meeting 2014
SIOP Meeting 2015
-
- International Society of Paediatric Oncology. 47th Congress of the International Society of Paediatric Oncology (SIOP) 2015 International Conference, 2015 Oct 8‐11; Cape Town, South Africa. Pediatric Blood & Cancer 2015;62(Suppl 4):S143‐418.
SIOP Meeting 2016
-
- International Society of Paediatric Oncology. 48th Congress of the International Society of Paediatric Oncology (SIOP) 2016 International Conference, 2016 Oct 19‐22; Dublin, Ireland. Pediatric Blood & Cancer 2016;63(Suppl 3):S5‐321. - PubMed
Sondak 2001
-
- Sondak VK, Chang AE. Clinical evaluation and treatment of soft tissue tumors. In: Weiss SW, Goldblum JR editor(s). Enzinger and Weiss's Soft Tissue Tumors. 4th Edition. St Louis: Mosby, 2001:21‐44.
Spunt 2006
-
- Spunt SL, Pappo AS. Childhood nonrhabdomyosarcoma soft tissue sarcomas are not adult‐type tumors. Journal of Clinical Oncology 2006;24(12):1958‐9. - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Therasse 2000
-
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. Journal of the National Cancer Institute 2000;92(3):205‐16. - PubMed
Tierney 2007
Tsao 2000
-
- Tsao H. Update on familial cancer syndromes and the skin. Journal of the American Academy of Dermatology 2000;42(6):939‐69. - PubMed
UICC 2009
-
- Sobin LH, Gospodarowicz MK, Wittekind C. The TNM Classification of Malignant Tumours. 7th Edition. Geneva: Union for International Cancer Control, 2009.
Weiss 2001
-
- Weiss SW, Goldblum JR. General considerations. In: Weiss SW, Goldblum JR editor(s). Enzinger and Weiss's Soft Tissue Tumors. 4th Edition. St Louis: Mosby, 2001:1‐19.
Woods 1999
-
- Woods WG. Myeloablative therapy followed by stem cell rescue for pediatric solid tumors: a non‐transplanter's perspective. Cancer Research Therapy and Control 1999;9(1‐2):95‐9.
References to other published versions of this review
Peinemann 2010
-
- Peinemann F, Bartel C, Hildebrandt M, Kulig M, Burkhardt‐Hammer T, Kröger N, et al. Autologous stem cell transplantation following high‐dose chemotherapy for non‐rhabdomyosarcoma soft tissue sarcomas. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD008216] - DOI - PubMed
Peinemann 2011
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical