Multiparametric Quantitative Ultrasound Imaging in Assessment of Chronic Kidney Disease
- PMID: 28407281
- PMCID: PMC5640470
- DOI: 10.1002/jum.14209
Multiparametric Quantitative Ultrasound Imaging in Assessment of Chronic Kidney Disease
Abstract
Objectives: To evaluate the value of multiparametric quantitative ultrasound imaging in assessing chronic kidney disease (CKD) using kidney biopsy pathologic findings as reference standards.
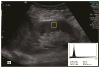
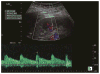
Methods: We prospectively measured multiparametric quantitative ultrasound markers with grayscale, spectral Doppler, and acoustic radiation force impulse imaging in 25 patients with CKD before kidney biopsy and 10 healthy volunteers. Based on all pathologic (glomerulosclerosis, interstitial fibrosis/tubular atrophy, arteriosclerosis, and edema) scores, the patients with CKD were classified into mild (no grade 3 and <2 of grade 2) and moderate to severe (at least 2 of grade 2 or 1 of grade 3) CKD groups. Multiparametric quantitative ultrasound parameters included kidney length, cortical thickness, pixel intensity, parenchymal shear wave velocity, intrarenal artery peak systolic velocity (PSV), end-diastolic velocity (EDV), and resistive index. We tested the difference in quantitative ultrasound parameters among mild CKD, moderate to severe CKD, and healthy controls using analysis of variance, analyzed correlations of quantitative ultrasound parameters with pathologic scores and the estimated glomerular filtration rate (GFR) using Pearson correlation coefficients, and examined the diagnostic performance of quantitative ultrasound parameters in determining moderate CKD and an estimated GFR of less than 60 mL/min/1.73 m2 using receiver operating characteristic curve analysis.
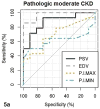
Results: There were significant differences in cortical thickness, pixel intensity, PSV, and EDV among the 3 groups (all P < .01). Among quantitative ultrasound parameters, the top areas under the receiver operating characteristic curves for PSV and EDV were 0.88 and 0.97, respectively, for determining pathologic moderate to severe CKD, and 0.76 and 0.86 for estimated GFR of less than 60 mL/min/1.73 m2 . Moderate to good correlations were found for PSV, EDV, and pixel intensity with pathologic scores and estimated GFR.
Conclusions: The PSV, EDV, and pixel intensity are valuable in determining moderate to severe CKD. The value of shear wave velocity in assessing CKD needs further investigation.
Keywords: Doppler ultrasound imaging; acoustic radiation force impulse; chronic kidney disease; quantitative ultrasound imaging; shear wave velocity.
© 2017 by the American Institute of Ultrasound in Medicine.
Figures









References
-
- El Nahas M. The global challenge of chronic kidney disease. Kidney Int. 2005;68:2918–2929. - PubMed
-
- Boffa JJ, Lu Y, Placier S, Stefanski A, Dussaule JC, Chatziantoniou C. Regression of renal vascular and glomerular fibrosis: role of angiotensin II receptor antagonism and matrix metalloproteinases. J Am Soc Nephrol. 2003;14:1132–1144. - PubMed
-
- Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

