Global Bi-ventricular endocardial distribution of activation rate during long duration ventricular fibrillation in normal and heart failure canines
- PMID: 28407744
- PMCID: PMC5390480
- DOI: 10.1186/s12872-017-0530-5
Global Bi-ventricular endocardial distribution of activation rate during long duration ventricular fibrillation in normal and heart failure canines
Abstract
Background: The objective of this study was to detect differences in the distribution of the left and right ventricle (LV & RV) activation rate (AR) during short-duration ventricular fibrillation (SDVF, <1 min) and long-duration ventricular fibrillation VF (LDVF, >1 min) in normal and heart failure (HF) canine hearts.
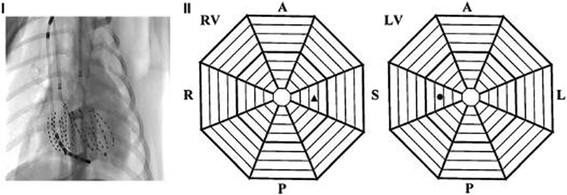
Methods: Ventricular fibrillation (VF) was electrically induced in six healthy dogs (control group) and six dogs with right ventricular pacing-induced congestive HF (HF group). Two 64-electrode basket catheters deployed in the LV and RV were used for global endocardium electrical mapping. The AR of VF was estimated by fast Fourier transform analysis from each electrode.
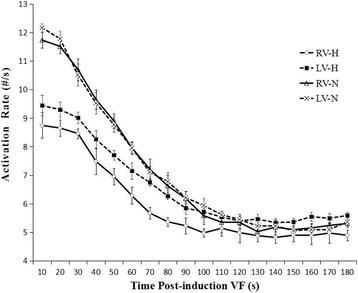
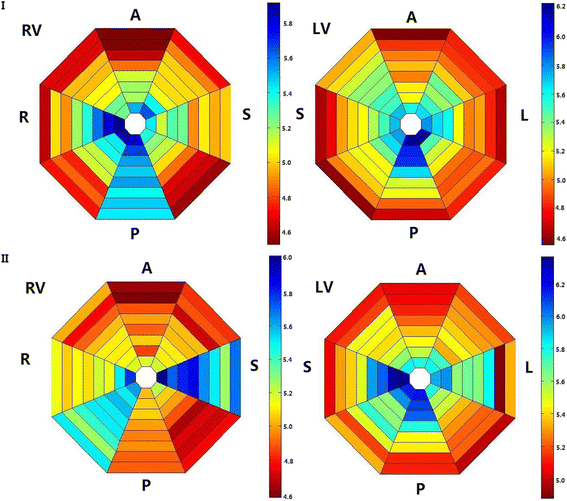
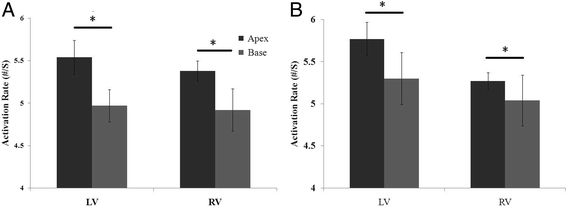
Results: In the control group, the LV was activated faster than the RV in the first 20 s, after which there was no detectable difference in the AR between them. When analyzing the distribution of the AR within the bi-ventricles at 3 min of LDVF, the posterior LV was activated fastest, while the anterior was slowest. In the HF group, a detectable AR gradient existed between the two ventricles within 3 min of VF, with the LV activating more quickly than the RV. When analyzing the distribution of the AR within the bi-ventricles at 3 min of LDVF, the septum of the LV was activated fastest, while the anterior was activated slowest.
Conclusions: A global bi-ventricular endocardial AR gradient existed within the first 20 s of VF but disappeared in the LDVF in healthy hearts. However, the AR gradient was always observed in both SDVF and LDVF in HF hearts. The findings of this study suggest that LDVF in HF hearts can be maintained differently from normal hearts, which accordingly should lead to the development of different management strategies for LDVF resuscitation.
Keywords: Activation Rate; Heart Failure; Ventricular Fibrillation.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

