Saline versus balanced crystalloids for intravenous fluid therapy in the emergency department: study protocol for a cluster-randomized, multiple-crossover trial
- PMID: 28407811
- PMCID: PMC5390477
- DOI: 10.1186/s13063-017-1923-6
Saline versus balanced crystalloids for intravenous fluid therapy in the emergency department: study protocol for a cluster-randomized, multiple-crossover trial
Abstract
Background: Prior studies in critically ill patients suggest the supra-physiologic chloride concentration of 0.9% ("normal") saline may be associated with higher risk of renal failure and death compared to physiologically balanced crystalloids. However, the comparative effects of 0.9% saline and balanced fluids are largely unexamined among patients outside the intensive care unit, who represent the vast majority of patients treated with intravenous fluids.
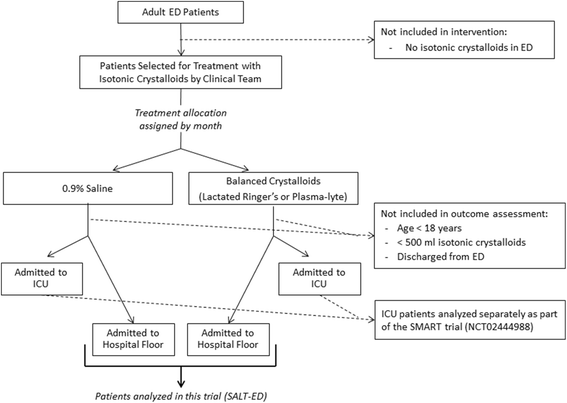
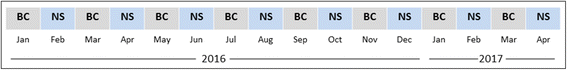
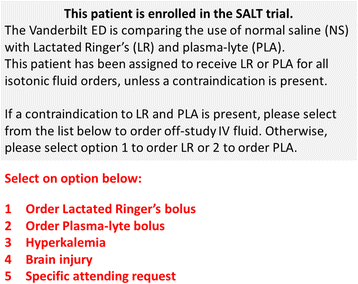
Methods/design: This study, entitled Saline Against Lactated Ringer's or Plasma-Lyte in the Emergency Department (SALT-ED), is a pragmatic, cluster, multiple-crossover trial at a single institution evaluating clinical outcomes of adults treated with 0.9% saline versus balanced crystalloids for intravenous fluid resuscitation in the emergency department. All adults treated in the study emergency department receiving at least 500 mL of isotonic crystalloid solution during usual clinical care and subsequently hospitalized outside the intensive care unit are included. Treatment allocation of 0.9% saline versus balanced crystalloids is assigned by calendar month, with study patients treated during the same month assigned to the same fluid type. The first month (January 2016) was randomly assigned to balanced crystalloids, with each subsequent month alternating between 0.9% saline and balanced crystalloids. For balanced crystalloid treatment, clinicians can choose either Lactated Ringer's or Plasma-Lyte A©. The study period is set at 16 months, which will result in an anticipated estimated sample size of 15,000 patients. The primary outcome is hospital-free days to day 28, defined as the number of days alive and out of the hospital from the index emergency department visit until 28 days later. Major secondary outcomes include proportion of patients who develop acute kidney injury by creatinine measurements; major adverse kidney events by hospital discharge or day 30 (MAKE30), which is a composite outcome of death, new renal replacement therapy, and persistent creatinine elevation >200% of baseline; and in-hospital mortality.
Discussion: This ongoing pragmatic trial will provide the most comprehensive evaluation to date of clinical outcomes associated with 0.9% saline compared to physiologically balanced fluids in patients outside the intensive care unit.
Trial registration: ClinicalTrials.gov, NCT02614040 . Registered on 18 November 2015.
Keywords: Crystalloid; Emergency department; Intravenous fluids; Pragmatic trial; Renal failure; Saline.
Figures
Similar articles
-
Clinical Effects of Balanced Crystalloids vs Saline in Adults With Diabetic Ketoacidosis: A Subgroup Analysis of Cluster Randomized Clinical Trials.JAMA Netw Open. 2020 Nov 2;3(11):e2024596. doi: 10.1001/jamanetworkopen.2020.24596. JAMA Netw Open. 2020. PMID: 33196806 Free PMC article. Clinical Trial.
-
Balanced crystalloids versus saline in the intensive care unit: study protocol for a cluster-randomized, multiple-crossover trial.Trials. 2017 Mar 16;18(1):129. doi: 10.1186/s13063-017-1871-1. Trials. 2017. PMID: 28302179 Free PMC article. Clinical Trial.
-
Balanced Crystalloids versus Saline in Noncritically Ill Adults.N Engl J Med. 2018 Mar 1;378(9):819-828. doi: 10.1056/NEJMoa1711586. Epub 2018 Feb 27. N Engl J Med. 2018. PMID: 29485926 Free PMC article. Clinical Trial.
-
Balanced Crystalloids Versus Saline in Critically Ill Adults: A Systematic Review and Meta-analysis.Ann Pharmacother. 2020 Jan;54(1):5-13. doi: 10.1177/1060028019866420. Epub 2019 Jul 31. Ann Pharmacother. 2020. PMID: 31364382
-
0.9% NaCl (Normal Saline) - Perhaps not so normal after all?Transfus Apher Sci. 2018 Feb;57(1):127-131. doi: 10.1016/j.transci.2018.02.021. Epub 2018 Feb 21. Transfus Apher Sci. 2018. PMID: 29523397 Free PMC article. Review.
Cited by
-
An early warning model for predicting major adverse kidney events within 30 days in sepsis patients.Front Med (Lausanne). 2024 Feb 26;10:1327036. doi: 10.3389/fmed.2023.1327036. eCollection 2023. Front Med (Lausanne). 2024. PMID: 38469459 Free PMC article.
-
An early warning model for predicting major adverse kidney events within 30 days in acute pancreatitis patients.Ren Fail. 2024 Dec;46(2):2424468. doi: 10.1080/0886022X.2024.2424468. Epub 2024 Dec 4. Ren Fail. 2024. PMID: 39632254 Free PMC article.
-
Feasibility of Extracting Meaningful Patient Centered Outcomes From the Electronic Health Record Following Critical Illness in the Elderly.Front Med (Lausanne). 2022 Jun 6;9:826169. doi: 10.3389/fmed.2022.826169. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35733861 Free PMC article.
-
The Impact of Normal Saline or Balanced Crystalloid on Plasma Chloride Concentration and Acute Kidney Injury in Patients With Predicted Severe Acute Pancreatitis: Protocol of a Phase II, Multicenter, Stepped-Wedge, Cluster-Randomized, Controlled Trial.Front Med (Lausanne). 2021 Oct 4;8:731955. doi: 10.3389/fmed.2021.731955. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34671619 Free PMC article.
-
Clinical Effects of Balanced Crystalloids vs Saline in Adults With Diabetic Ketoacidosis: A Subgroup Analysis of Cluster Randomized Clinical Trials.JAMA Netw Open. 2020 Nov 2;3(11):e2024596. doi: 10.1001/jamanetworkopen.2020.24596. JAMA Netw Open. 2020. PMID: 33196806 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

