Cell Death Pathway That Monitors Spore Morphogenesis
- PMID: 28408070
- PMCID: PMC5522370
- DOI: 10.1016/j.tim.2017.03.005
Cell Death Pathway That Monitors Spore Morphogenesis
Abstract
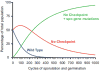
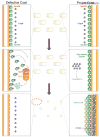
The use of quality control mechanisms to stall developmental pathways or completely remove defective cells from a population is a widespread strategy to ensure the integrity of morphogenetic programs. Endospore formation (sporulation) is a well conserved microbial developmental strategy in the Firmicutes phylum wherein a progenitor cell that faces starvation differentiates to form a dormant spore. Despite the conservation of this strategy, it has been unclear what selective pressure maintains the fitness of this developmental program, composed of hundreds of unique genes, during multiple rounds of vegetative growth when sporulation is not required. Recently, a quality control pathway was discovered in Bacillus subtilis which monitors the assembly of the spore envelope and specifically eliminates, through cell lysis, sporulating cells that assemble the envelope incorrectly. Here, we review the use of checkpoints that govern the entry into sporulation in B. subtilis and discuss how the use of regulated cell death pathways during bacterial development may help maintain the fidelity of the sporulation program in the species.
Keywords: SpoIVA; SpoVM; apoptosis; gametogenesis; lamins; nuclear envelope; programmed cell death.
Published by Elsevier Ltd.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

