5 Year Expression and Neutrophil Defect Repair after Gene Therapy in Alpha-1 Antitrypsin Deficiency
- PMID: 28408179
- PMCID: PMC5474959
- DOI: 10.1016/j.ymthe.2017.03.029
5 Year Expression and Neutrophil Defect Repair after Gene Therapy in Alpha-1 Antitrypsin Deficiency
Abstract
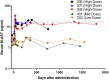
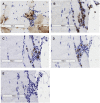
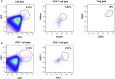
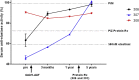
Alpha-1 antitrypsin deficiency is a monogenic disorder resulting in emphysema due principally to the unopposed effects of neutrophil elastase. We previously reported achieving plasma wild-type alpha-1 antitrypsin concentrations at 2.5%-3.8% of the purported therapeutic level at 1 year after a single intramuscular administration of recombinant adeno-associated virus serotype 1 alpha-1 antitrypsin vector in alpha-1 antitrypsin deficient patients. We analyzed blood and muscle for alpha-1 antitrypsin expression and immune cell response. We also assayed previously reported markers of neutrophil function known to be altered in alpha-1 antitrypsin deficient patients. Here, we report sustained expression at 2.0%-2.5% of the target level from years 1-5 in these same patients without any additional recombinant adeno-associated virus serotype-1 alpha-1 antitrypsin vector administration. In addition, we observed partial correction of disease-associated neutrophil defects, including neutrophil elastase inhibition, markers of degranulation, and membrane-bound anti-neutrophil antibodies. There was also evidence of an active T regulatory cell response (similar to the 1 year data) and an exhausted cytotoxic T cell response to adeno-associated virus serotype-1 capsid. These findings suggest that muscle-based alpha-1 antitrypsin gene replacement is tolerogenic and that stable levels of M-AAT may exert beneficial neutrophil effects at lower concentrations than previously anticipated.
Keywords: A1AT; AAT; AAV; PD-1; Tregs; alpha-1 antitrypsin; clinical trial; exhausted T cells; gene therapy; rAAV.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures




References
-
- de Serres F., Blanco I. Role of alpha-1 antitrypsin in human health and disease. J. Intern. Med. 2014;276:311–335. - PubMed
-
- Larsson C. Natural history and life expectancy in severe alpha1-antitrypsin deficiency, Pi Z. Acta Med. Scand. 1978;204:345–351. - PubMed
-
- Browne R.J., Mannino D.M., Khoury M.J. Alpha 1-antitrypsin deficiency deaths in the United States from 1979-1991. An analysis using multiple-cause mortality data. Chest. 1996;110:78–83. - PubMed
-
- Aliño S.F., Crespo J., Bobadilla M., Lejarreta M., Blaya C., Crespo A. Expression of human alpha 1-antitrypsin in mouse after in vivo gene transfer to hepatocytes by small liposomes. Biochem. Biophys. Res. Commun. 1994;204:1023–1030. - PubMed
-
- Aliño S.F., Bobadilla M., Crespo J., Lejarreta M. Human alpha 1-antitrypsin gene transfer to in vivo mouse hepatocytes. Hum. Gene Ther. 1996;7:531–536. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

