Synergistic Immunostimulatory Effects and Therapeutic Benefit of Combined Histone Deacetylase and Bromodomain Inhibition in Non-Small Cell Lung Cancer
- PMID: 28408401
- PMCID: PMC5540748
- DOI: 10.1158/2159-8290.CD-16-1020
Synergistic Immunostimulatory Effects and Therapeutic Benefit of Combined Histone Deacetylase and Bromodomain Inhibition in Non-Small Cell Lung Cancer
Abstract
Effective therapies for non-small cell lung cancer (NSCLC) remain challenging despite an increasingly comprehensive understanding of somatically altered oncogenic pathways. It is now clear that therapeutic agents with potential to impact the tumor immune microenvironment potentiate immune-orchestrated therapeutic benefit. Herein, we evaluated the immunoregulatory properties of histone deacetylase (HDAC) and bromodomain inhibitors, two classes of drugs that modulate the epigenome, with a focus on key cell subsets that are engaged in an immune response. By evaluating human peripheral blood and NSCLC tumors, we show that the selective HDAC6 inhibitor ricolinostat promotes phenotypic changes that support enhanced T-cell activation and improved function of antigen-presenting cells. The bromodomain inhibitor JQ1 attenuated CD4+FOXP3+ T regulatory cell suppressive function and synergized with ricolinostat to facilitate immune-mediated tumor growth arrest, leading to prolonged survival of mice with lung adenocarcinomas. Collectively, our findings highlight the immunomodulatory effects of two epigenetic modifiers that, together, promote T cell-mediated antitumor immunity and demonstrate their therapeutic potential for treatment of NSCLC.Significance: Selective inhibition of HDACs and bromodomain proteins modulates tumor-associated immune cells in a manner that favors improved T-cell function and reduced inhibitory cellular mechanisms. These effects facilitated robust antitumor responses in tumor-bearing mice, demonstrating the therapeutic potential of combining these epigenetic modulators for the treatment of NSCLC. Cancer Discov; 7(8); 852-67. ©2017 AACR.This article is highlighted in the In This Issue feature, p. 783.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures
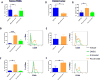

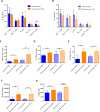
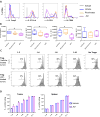
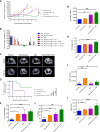

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

