Posterior Parietal Cortex Guides Visual Decisions in Rats
- PMID: 28408414
- PMCID: PMC5426183
- DOI: 10.1523/JNEUROSCI.0105-17.2017
Posterior Parietal Cortex Guides Visual Decisions in Rats
Abstract
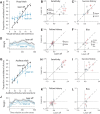
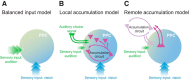
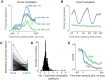
Neurons in putative decision-making structures can reflect both sensory and decision signals, making their causal role in decisions unclear. Here, we tested whether rat posterior parietal cortex (PPC) is causal for processing visual sensory signals or instead for accumulating evidence for decision alternatives. We disrupted PPC activity optogenetically during decision making and compared effects on decisions guided by auditory versus visual evidence. Deficits were largely restricted to visual decisions. To further test for visual dominance in PPC, we evaluated electrophysiological responses after individual sensory events and observed much larger response modulation after visual stimuli than auditory stimuli. Finally, we measured trial-to-trial spike count variability during stimulus presentation and decision formation. Variability decreased sharply, suggesting that the network is stabilized by inputs, unlike what would be expected if sensory signals were locally accumulated. Our findings suggest that PPC plays a causal role in processing visual signals that are accumulated elsewhere.SIGNIFICANCE STATEMENT Defining the neural circuits that support decision making bridges a gap between our understanding of simple sensorimotor reflexes and our understanding of truly complex behavior. However, identifying brain areas that play a causal role in decision making has proved challenging. We tested the causal role of a candidate component of decision circuits, the rat posterior parietal cortex (PPC). Our interpretation of the data benefited from our use of animals trained to make decisions guided by either visual or auditory evidence. Our results suggest that PPC plays a causal role specifically in visual decision making and may support sensory aspects of the decision, such as interpreting the visual signals so that evidence for a decision can be accumulated elsewhere.
Keywords: decision making.
Copyright © 2017 the authors 0270-6474/17/374954-13$15.00/0.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
