The Membrane Transport System of the Guard Cell and Its Integration for Stomatal Dynamics
- PMID: 28408539
- PMCID: PMC5462021
- DOI: 10.1104/pp.16.01949
The Membrane Transport System of the Guard Cell and Its Integration for Stomatal Dynamics
Abstract
Stomatal guard cells are widely recognized as the premier plant cell model for membrane transport, signaling, and homeostasis. This recognition is rooted in half a century of research into ion transport across the plasma and vacuolar membranes of guard cells that drive stomatal movements and the signaling mechanisms that regulate them. Stomatal guard cells surround pores in the epidermis of plant leaves, controlling the aperture of the pore to balance CO2 entry into the leaf for photosynthesis with water loss via transpiration. The position of guard cells in the epidermis is ideally suited for cellular and subcellular research, and their sensitivity to endogenous signals and environmental stimuli makes them a primary target for physiological studies. Stomata underpin the challenges of water availability and crop production that are expected to unfold over the next 20 to 30 years. A quantitative understanding of how ion transport is integrated and controlled is key to meeting these challenges and to engineering guard cells for improved water use efficiency and agricultural yields.
© 2017 The author(s). All Rights Reserved.
Figures
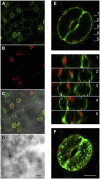
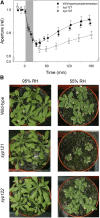
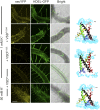






References
-
- Accardi A, Miller C (2004) Secondary active transport mediated by a prokaryotic homologue of ClC Cl- channels. Nature 427: 803–807 - PubMed
-
- Acharya BR, Jeon BW, Zhang W, Assmann SM (2013) Open Stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells. New Phytol 200: 1049–1063 - PubMed
-
- Ache P, Becker D, Ivashikina N, Dietrich P, Roelfsema MRG, Hedrich R (2000) GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Lett 486: 93–98 - PubMed
-
- Alexandre J, Lassalles JP, Kado RT (1990) Opening of Ca2+ channels in isolated red beet root vacuole membrane by inositol 1,4,5-trisphosphate. Nature 343: 567–570
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

