Architecture of a transcribing-translating expressome
- PMID: 28408604
- PMCID: PMC5528865
- DOI: 10.1126/science.aal3059
Architecture of a transcribing-translating expressome
Abstract
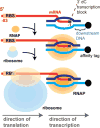
DNA transcription is functionally coupled to messenger RNA (mRNA) translation in bacteria, but how this is achieved remains unclear. Here we show that RNA polymerase (RNAP) and the ribosome of Escherichia coli can form a defined transcribing and translating "expressome" complex. The cryo-electron microscopic structure of the expressome reveals continuous protection of ~30 nucleotides of mRNA extending from the RNAP active center to the ribosome decoding center. The RNAP-ribosome interface includes the RNAP subunit α carboxyl-terminal domain, which is required for RNAP-ribosome interaction in vitro and for pronounced cell growth defects upon translation inhibition in vivo, consistent with its function in transcription-translation coupling. The expressome structure can only form during transcription elongation and explains how translation can prevent transcriptional pausing, backtracking, and termination.
Copyright © 2017, American Association for the Advancement of Science.
Figures
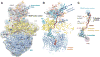
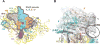

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

